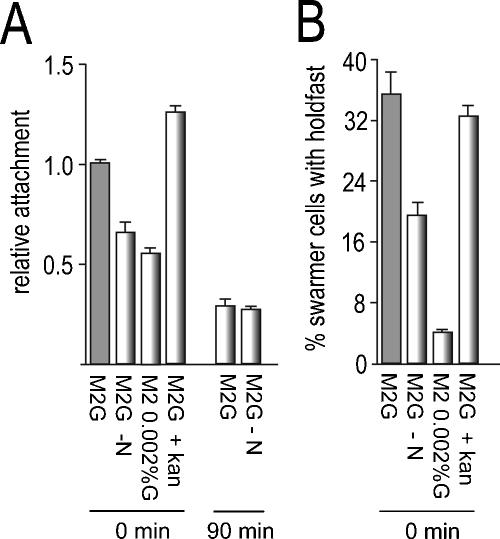
FIG. 2.
Holdfast formation and optimal surface attachment requires SW cell development but not de novo protein synthesis. (A) Purified SW cells of C. crescentus CB15 wild type (ATCC 19089) were released into glucose minimal medium (M2G), M2G lacking nitrogen (M2G −N), M2 with a 100-fold-reduced glucose concentration (M2 0.002%G), and M2G containing kanamycin (50 μg/ml; the MIC of kanamycin for C. crescentus is 1 μg/ml). Culture aliquots were immediately (0 min) transferred to microtiter plates and allowed to attach to the plastic surface for 30 min. Attachment was quantified as described in the legend to Fig. 1. As a control, purified SW cells were released into M2G and allowed to go through the SW-to-ST cell transition for 90 min (90 min) before cells were harvested, washed, and released into either M2G or M2G lacking nitrogen (M2G −N). The cells were transferred to microtiter plates and allowed to attach to the plastic surface for 30 min. (B) Purified SW cells were treated as in panel A and incubated for 30 min at 30°C, and the fraction of cells with a visible holdfast was determined by fluorescent labeling. The error bars indicate standard deviations of the mean of triplicate experiments.