Abstract
The pst operon of Clostridium acetobutylicum ATCC 824 comprises five genes, pstS, pstC, pstA, pstB, and phoU, and shows a gene architecture identical to that of Escherichia coli. Deduced proteins are predicted to represent a high-affinity phosphate-specific ABC (ATP-binding cassette) transport system (Pst) and a protein homologous to PhoU, a negative phosphate regulon regulator. We analyzed the expression patterns of the pst operon in Pi-limited chemostat cultures during acid production at pH 5.8 or solvent production at pH 4.5 and in response to Pi pulses. Specific mRNA transcripts were found only when external Pi concentrations had dropped below 0.2 mM. Two specific transcripts were detected, a 4.7-kb polycistronic mRNA spanning the whole operon and a quantitatively dominating 1.2-kb mRNA representing the first gene, pstS. The mRNA levels clearly differed depending on the external pH. The amounts of the full-length mRNA detected were about two times higher at pH 5.8 than at pH 4.5. The level of pstS mRNA increased by a factor of at least 8 at pH 5.8 compared to pH 4.5 results. Primer extension experiments revealed only one putative transcription start point 80 nucleotides upstream of pstS. Thus, additional regulatory sites are proposed in the promoter region, integrating two different extracellular signals, namely, depletion of inorganic phosphate and the pH of the environment. After phosphate pulses were applied to a phosphate-limited chemostat we observed faster phosphate consumption at pH 5.8 than at pH 4.5, although higher optical densities were recorded at pH 4.5.
The ability to respond and to adapt to hostile environmental conditions is fundamental for bacterial survival in nature. Among several circumstances causing “stress” the limited availability of substrates or bioelements plays a crucial role. In soil, one of the natural habitats of Clostridium acetobutylicum, phosphorus is expected to be a major growth-limiting bioelement (7, 16, 46). Hence, to cope with phosphate limitation microorganisms have evolved complex regulatory systems to assimilate inorganic phosphate (Pi) efficiently from the environment (16, 46).
In the gram-positive bacterium C. acetobutylicum, a strict anaerobe, limitation of phosphate in a chemostat culture in combination with excess of glucose and a pH below 5 reproducibly triggers a fundamental change of the metabolism from the production of organic acids (acetate and butyrate) to solvents (butanol and acetone) as main fermentation products (6, 11). In a batch culture, this metabolic switch is linked to other particular features of the clostridial cell cycle such as the formation of endospores (26), morphological changes (motility, cell shape), and synthesis of granulose (39). The limitation of phosphate seems to be at least one important factor in this complex regulation network (25). Even though these data have been known for nearly 20 years nothing is known about the phosphate-dependent gene regulation in C. acetobutylicum, e.g., the existence of a clostridial Pho regulon.
Phosphate (Pho) regulons of bacteria include all genes whose expression rates respond to phosphate limitation initially controlled by a two-component regulatory system. For example, in Escherichia coli the Pho regulon comprises at least 38 different genes (46) and in B. subtilis at least 31 genes (4, 16). Among these a high-affinity phosphate uptake system as described for several bacterial species such E. coli (1, 38), B. subtilis (37), Mycobacterium tuberculosis (9), Streptococcus pneumoniae (30), or Pseudomonas aeruginosa (28) can usually be found.
We have started to analyze the molecular response of C. acetobutylicum to phosphate limitation. Here, we report on the characterization and the transcriptional analysis of the pst operon as the first gene locus of C. acetobutylicum which seems to be a member of a putative Pho regulon. In addition to the external phosphate concentration, the pH value, which is an important factor for product formation in C. acetobutylicum (acid or solvent production), also influenced the transcript levels of the pst operon.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
C. acetobutylicum ATCC 824 (laboratory collection) was grown under anaerobic conditions at 37°C. Precultures (10 ml) were inoculated with 107 to 108 spores and pasteurized for 5 min at 75°C before incubation. Phosphate-limited chemostat experiments were performed as described by Bahl et al. (6) in a meredos 1.2-l fermenter system (Nörten-Hardenberg, Germany) or a BiostatB 1.5-l fermenter system (BBI, Melsungen, Germany) with 0.5 mM KH2PO4 and 4% (wt/vol) glucose in the supplying medium. External pH was adjusted to constant values (pH 4.5 or pH 5.8) by automatic addition of 2 M KOH. For the generation of a phosphate surplus a sterile solution of 1 M KH2PO4 was injected into the culture vessel, leading to final concentrations of 10 mM Pi.
Measurement of OD.
The optical density at 600 nm (OD600) was determined in a spectrophotometer (Spekol 1100 photometer; Analytic Jena, Jena, Germany) using 1.5-ml plastic cuvettes with a light path of 1 cm. Samples out of the chemostat culture were diluted with distilled water to an OD below 0.3 and measured against distilled water.
Measurement of phosphate concentration.
Phosphate was measured colorimetrically in form of molybdate-vanadate complexes (50). Therefore, 0.5-ml samples of cell-free culture supernatants (see “Analysis of fermentation products” below) were deproteinated by mixing with an equal volume of 1.2 M trichloroacetic acid, incubated for 15 min at room temperature, and centrifuged (16,000 × g for 15 min at room temperature). Addition of 0.5 ml 21 mM ammonium-vanadate (in 0.28 M HNO3) and 0.5 ml 40 mM ammonium-molybdate (in 1.25 M H2SO4) to 0.5 ml of the deproteinated supernatant was followed by vortexing and incubation for 10 min at room temperature. Extinction (E405) of the yellow colored complexes was measured at 405 nm (Ultrospec 3000 photometer; Amersham Pharmacia Biotech, Freiburg, Germany). The slope (m) of a calibration curve between 0.1 and 1 mM KH2PO4 was used for the calculation of the phosphate concentrations (Cphosphate) by use of the equation Cphosphate [in millimoles] = E405/m.
Analysis of fermentation products.
Samples (2 ml) of C. acetobutylicum cell suspensions were collected and quickly sedimented at 16,000 × g for 30 s at 4°C in a benchtop centrifuge. Further treatment of cell-free supernatants and the detection of the fermentation products (acetate, acetone, butanol, butyrate, and ethanol) by use of a gas chromatograph (CP9001; Chrompack, Frankfurt am Main, Germany) and a Chromosorb 101 (80/100 mesh) column were performed by the method of Thormann et al. (44).
DNA isolation and manipulation.
For the isolation of chromosomal DNA C. acetobutylicum was grown in batch cultures with 2× YTG medium supplemented with 0.4% (wt/vol) glucose (33). At an OD of 1.2, cell aliquots of 100 ml were harvested by centrifugation (4,500 × g for 10 min at 4°C) and washed three times with 10 ml Tris-acetate-EDTA buffer containing 10% (wt/vol) sucrose. The resulting pellets were stored at −20°C until they were suspended homogenously in 3.8 ml Tris-acetate-EDTA-sucrose buffer, mixed with 1 ml lysozyme-RNase solution (20 mg/ml lysozyme-2 mg/ml DNase-free RNase [Applichem, Darmstadt, Germany], a stock solution according to Sambrook and Russell [40], in 100 mM Tris-HCl-15 mM NaCl [pH 7.5]), and incubated at 37°C for 30 min. After successive addition of 500 μl 0.5 M EDTA (pH 8.0), 40 μl Tris-HCl (pH 8.0), and 30 μl 10% (wt/vol) sodium dodecyl sulfate (SDS), the mixed suspension was kept for 10 min at 37°C before 500 μl proteinase K (Applichem, Darmstadt, Germany) (20 mg/ml) was added, and incubation proceeded for another 2 h. DNA was extracted by addition of 1.4 ml 5 M Na-perchlorate and treatment with chloroform-isoamylalcohol three times according to standard protocols. Precipitation of the DNA was performed by mixing with 1 volume of isopropanol for 5 min at room temperature and centrifugation (16,000 × g for 20 min at 4°C). The air-dried DNA was dissolved in 2 ml of Tris-EDTA (TE) buffer (pH 8.0), 200 μl of proteinase K solution was added, and the solution was incubated overnight at 37°C. Subsequently, the volume was adjusted to 4 ml with distilled water, and 600 μl 3 M sodium acetate (pH 5.2) as added followed by extraction with chloroform-isoamylalcohol three times. DNA was precipitated as described above, desalted by washing with ethanol (70% [vol/vol]), and dissolved in 200 μl of TE buffer.
Isolation of total RNA.
Total RNA of C. acetobutylicum was isolated using a modified hot phenol protocol based on the method of Oelmüller et al. (32). In brief, cells of 2-ml culture aliquots (in 2-ml Eppendorf tubes) were harvested as quickly as possible as described above (see “Analysis of fermentation products”). After the supernatant was discarded, cell pellets were shock frosted in liquid nitrogen and stored at −70°C until use. For the isolation of RNA, 1.2 ml of acidic phenol (Applichem, Darmstadt, Germany) (pH 4.5) and 15 μl 25% (wt/vol) SDS were mixed and heated to 65°C. Frozen cell pellets were suspended in 600 μl of ice-cold AE buffer (20 mM sodium acetate, 1 mM EDTA [pH 7.5]), immediately transferred into the hot SDS-phenol solution, and incubated for further 10 min at 65°C with repeated mixing. Phase separation was reached by centrifugation (6,000 × g for 15 min at 4°C). RNA extraction out of the upper aqueous phase was achieved by a repeated treatment with 600 μl of acidic phenol and 100 μl 2 M sodium acetate (pH 5.2) followed by precipitation with 2.5 volumes of ice-cold ethanol (96% [vol/vol]) for 2 h at −20°C and centrifugation for 1 h at 16,000 × g and 4°C. After being desalted by washing with 70% (vol/vol) ethanol the air-dried RNA pellets were dissolved in 200 μl of DNase buffer (40 mM Tris-HCl, 6 mM MgCl2·6 H2O, pH 5.5) with 50 U of RNase-free DNase (Amersham Pharmacia Biotech, Freiburg, Germany) and incubated for 30 min at 37°C. The RNA was purified by addition of 15 μl 2 M sodium acetate, an additional treatment with phenol, and precipitation with ethanol as described above. The dried RNA was dissolved in 20 μl of TE buffer. Quality was controlled by agarose gel electrophoresis, and quantity was measured in a spectrophotometer (Ultrospec3000; Amersham Pharmacia Biotech, Freiburg, Germany) at 260 nm.
Northern hybridization.
Probes for the Northern hybridization were PCR generated using standard protocols, Pwo polymerase (Peqlab, Biotechnology, Erlangen, Germany), and chromosomal DNA of C. acetobutylicum as a template. Oligonucleotides yqgG-1 and yqgG-2 (Table 1) were used for the generation of a pstS-specific 407-bp DNA fragment, pstB-1 and pstB-2 for the amplification of an internal 436-bp fragment of pstB, and worf-1 and worf-2 for a 397-bp fragment corresponding to cac1710 (phoU). Labeling with digoxigenin (DIG) (DIG DNA labeling and detection kit; Roche Diagnostic, Mannheim, Germany) and Northern blot hybridization at 42°C (overnight) were performed according to the directions in the manual of the manufacturer. Detection of chemiluminescence was performed as described previously (24).
TABLE 1.
Oligonucleotides
| Primer | Oligonucleotide sequence (5′ → 3′)a | Use(s) |
|---|---|---|
| yqgG-1 | TGGAAATTCAAATTCAAGCAGC | 5′pstS probe, 5′pstS RT-PCR |
| yqgG-2 | ATGTTTATTGCTTCACTTGGTC | 3′pstS probe, 3′pstS RT-PCR |
| pstB-1 | TATTATGGAAATGTTCAGGC | 5′pstB probe |
| pstB-2 | CAATACATAATCTTTGCTGC | 3′pstB probe |
| worf-1 | AAAGAATATGATGAAAAGCTGG | 5′CAC1710 probe |
| worf-2 | TCTTCTCCACTATTTATTCCAG | 3′CAC1710 probe |
| yqgG-PE-2 | IRD800-GAACATTAACACTCACATCGGG | Primer extension and |
| yqgG-PE-3 | IRD800-ATGGTTGAAGTGCTGTAGATCC | sequencing reaction of pstS |
| vor-yqgG-Eco2 | ATTATAAAgaattcATAGCTGCTG | Cloning of pstS gene region |
| nach-yqgG-Eco3 | TCCATAGCTTgaattcAATTTAAG | into pBluescript II SK(+) |
| yqgG-a | TTAGTGAAAGTTGCTCTTGTTC | pstS cDNA |
| yqgG-3 | TTAGGTTTTGAATTTAGTTACC | 5′pstS RT-PCR |
| yqgG-4 | GTAAGTTTAGATTGTAAAGATC | 5′pstS RT-PCR |
| yqgG-8 | TACTTTGGAGGTATTTTAAATG | 5′pstS RT-PCR |
| yqgG-9 | TAACATGTTAACTGTAAAGAGG | 5′pstS RT-PCR |
| yqgG-10 | AATTAACATTAGTATAATACTC | 5′pstS RT-PCR |
| RT-ptb-a | GTCTTATACATTACATTTCCAG | ptb-cDNA |
| RT-ptb-1 | TTTGGCATTAAGAAGATATCAG | 5′ptb RT-PCR |
| RT-ptb-2 | GCATATTAAACAAAGAAGTTGG | 3′ptb RT-PCR |
Lowercase letters indicate nucleotide substitutions used to generate restriction sites in the PCR products.
Primer extension analysis.
The infrared dye (IRD800)-labeled primer (MWG-Biotech, Ebersberg, Germany) (2 pmol) yqgG-PE-2 or yqgG-PE-3 (Table 1) was incubated for 10 min at 70°C together with 10 μg of total RNA and 12.5 U of human placenta RNase inhibitor (Amersham Pharmacia Biotech, Freiburg, Germany) in a total volume of 11 μl and chilled on ice. After addition of 4 μl of 5× first-strand synthesis buffer (Invitrogen, Karlsruhe, Germany), 2 μl of dithiothreitol (0.1 M), and 1 μl of deoxynucleoside triphosphate mix (Amersham Pharmacia Biotech, Freiburg, Germany) (10 mM), the solution was mixed and preincubated for 2 min at 42°C. Superscript RNase H− (Invitrogen, Karlsruhe, Germany) (200 U) was added, and reverse transcription was performed for 45 min at 42°C. The resulting RNA-primer extended hybrids were phenol-chloroform-isoamylalcohol extracted, precipitated with ethanol, air dried, and dissolved in 4 μl TE buffer. A 2 μl volume was mixed with 2 μl Stop solution and analyzed on a denaturing sequencing polyacrylamide gel on a LI-COR 4200 sequencer according to the instructions of the manufacturer (MWG-Biotech, Ebersberg, Germany). Parallel sequencing reactions (41) with the same primers and plasmid DNA (pUM8) as the template were separated on the same gel. pUM8 is a derivate of pBluescript II SK(+) (Stratagene, Amsterdam, Netherlands) containing an EcoRI-trimmed 1,417-bp PCR fragment which was generated using the primers vor-yqgG-Eco2′ and nach-yqgG-Eco3′ (Table 1) spanning the pstS gene and its upstream promoter region.
RT-PCR.
To ensure that all traces of DNA were degraded, 1 μg of total RNA was combined with 10,000 U RNase-free fast protein liquid chromatography pure DNase I, 5 U of Placenta RNase inhibitor (both from Amersham Pharmacia Biotech, Freiburg, Germany), and 4 μl of 5× first-strand synthesis buffer in a total volume of 16 μl and incubated at 42°C for 30 min. Subsequently, two-step reverse transcriptase PCR (RT-PCR) experiments were performed using a Reverse-iT first-strand synthesis kit and Ready-Load PCR mix (both from Advanced Biotechnologies Ltd., Epsom, United Kingdom) according to the instructions of the manufacturer. Gene-specific cDNA molecules were generated in the first step which served as templates in the following PCRs using RT-PCR primers (Table 1). For negative controls, experiments were done using water instead of RT.
RESULTS
Observations of a phosphate-limited chemostat of C. acetobutylicum after phosphate pulses.
For our investigations of the molecular response of C. acetobutylicum to phosphate limitation we used cells from a phosphate-limited (0.5 mM phosphate in the medium reservoir, 0 mM in the cell-free supernatant of the culture) chemostat. The cells were harvested either at steady state or after a phosphate pulse, e.g., in the time period between addition of phosphate (10 mM KH2PO4) and depletion again of phosphate due to the consumption by the bacteria and the dilution in the chemostat. Furthermore, the experiments were carried out at two different pH values. At steady state, mainly acids were produced at pH 5.8 and mainly solvents at pH 4.5 (Table 2).
TABLE 2.
Characteristic features of C. acetobutylicum growing under steady-state conditions in a phosphate-limited chemostat at pH 4.5 or pH 5.8
| Parameter | Solvent production | Acid production |
|---|---|---|
| Dilution rate | 0.075 h−1 | 0.075 h−1 |
| pH | 4.5 | 5.8 |
| Pi (medium reservoir) | 0.5 mM | 0.5 mM |
| Pi (culture vessel) | 0 mM | 0 mM |
| Acetone | 23 mM | 0.2 mM |
| Butanol | 33 mM | 2 mM |
| Ethanol | 4 mM | 3 mM |
| Acetate | 12 mM | 28 mM |
| Butyrate | 13 mM | 44 mM |
| OD600 | 4.8 | 4.1 |
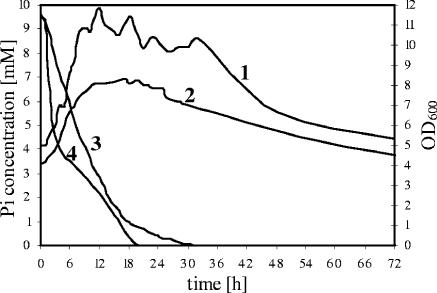
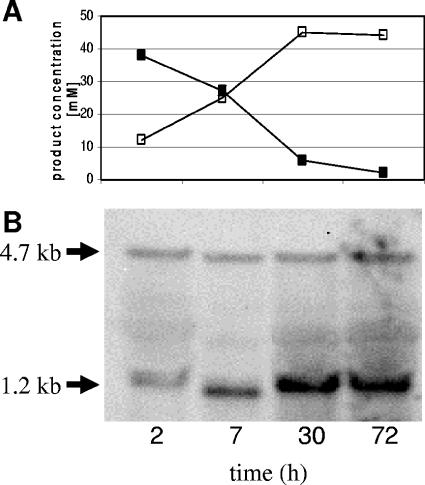
After phosphate pulses under these two different sets of growth conditions the following observations were made (Fig. 1). First of all, the OD increased at both pH values, proving that the chemostat had been running under growth-limiting phosphate concentration conditions prior to the pulse of Pi. However, the OD600 at pH 4.5 increased 2.4-fold (from 4.8 to 11.4) compared to 2-fold (from 4.1 to 8.3) at pH 5.8 (Fig. 1). On the other hand, the decrease of the phosphate concentration was faster at pH 5.8, especially in the first 10 h of the experiments. Thus, phosphate was detectable in the supernatant of the culture for 32 h after the pulse at pH 4.5 and for only 23 h at pH 5.8. Since in both cases the dilution rate was fixed at 0.075 h−1, this difference, which was observed in at least five independent experiments, was due to different results with respect to phosphate consumption by the bacteria. In accordance with the higher cell densities present after the phosphate pulse, increasing amounts of acids (pH 5.8) or solvents (pH 4.5) were produced (data not shown).
FIG. 1.
Effect of phosphate pulses (10 mM) on growth and phosphate consumption of C. acetobutylicum in a phosphate-limited chemostat. The results for the 72-h time period after the pulse in a representative experiment are shown. Basically the same results were obtained in five independent experiments. Line 1, OD at pH 4.5; line 2, OD at pH 5.8; line 3, phosphate concentrations at pH 4.5; line 4, phosphate concentrations at pH 5.8, a temperature of 37°C, and dilution rate of 0.075 h−1.
The pst operon of C. acetobutylicum.
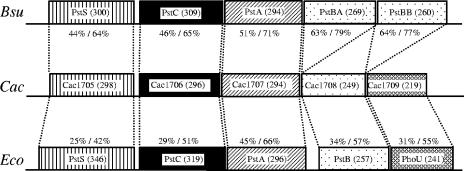
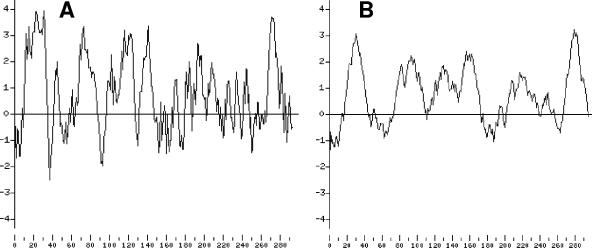
As a first target for our molecular analysis of the response of C. acetobutylicum to phosphate limitation we chose the pst operon, which represents a common member of Pho regulons in several other bacteria and is generally highly positively regulated at the transcriptional level after depletion of Pi in the environment. Usually, the pst operon encodes at least four different polypeptides or protein domains of a phosphate-specific ABC transport system (3). PstB (ATP binding), PstA, and PstC (channel domains, permeases) are membrane-bound components, whereas PstS is a membrane-associated (periplasmic) high-affinity phosphate-specific binding protein. In some microorganisms such as E. coli an additional open reading frame (phoU) encodes a so-called phosphate regulon regulator (42, 46). BLAST searches (2) of the B. subtilis PstS protein (BSU24990, 300 amino acids) (43) against the genome of C. acetobutylicum (29) revealed a striking similarity (44% identical, 64% similar amino acid residues) to the gene product (298 amino acids) of the open reading frame cac1705 (Fig. 2). This putative pstS gene is located on the leading strand starting nearly in the middle of the 3.94-Mbp chromosome at position 1,853,987. Downstream of pstS four open reading frames (cac1706 to cac1709) are located with the same direction of transcription. They all start with an ATG codon and are preceded by putative ribosome binding sites, each indicating individual translation (29). Since all their deduced gene products reveal high similarities not only to proteins of ABC transport systems in general but specifically to phosphate specific-uptake systems (Fig. 2) they were named PstC, PstA, PstB, and PhoU in accordance with their designated counterparts in E. coli (46) and B. subtilis (43). The assumption that PstC and PstA (Cac1706 and Cac1707) represent inner membrane components is supported by hydropathicity plot analyses (20) revealing their strong hydrophobic character (Fig. 3).
FIG. 2.
Comparison of the pst operons of C. acetobutylicum, E. coli, and B. subtilis. Boxes symbolize relative locations and sizes of the corresponding genes. Identical illustrations indicate proteins of equal functions, and numbers in parentheses represent amino acid residues. Percent values inform about the identity and similarity of the polypeptides of C. acetobutylicum (Cac) to their equivalents in E. coli (Eco) or B. subtilis (Bsu). In B. subtilis, the ATP-binding protein consists of two different subunits, PstBA and PstBB.
FIG. 3.
Hydropathicity plot of PstC (A) and PstA (B) of C. acetobutylicum. The x coordinates correspond to amino acid numbers; the y coordinates show hydrophobic (positive) and hydrophilic (negative) characteristics (20).
The pst operon of C. acetobutylicum obviously includes the genetic information for a PhoU-like protein encoded by cac1709. It showed highest similarities with PhoU of Moorella thermoacetica (45% identical, 66% similar amino acids) followed by several other PhoU-like proteins (data not shown). Thus, the gene architecture of the pst operon of C. acetobutylicum ATCC 824 is identical to that of E. coli but interestingly quite different from the more closely related B. subtilis.
Transcriptional analysis of the pst operon of C. acetobutylicum: general aspects.
To evaluate the transcription pattern of the pst operon, total RNA samples of C. acetobutylicum cells grown in a phosphate-limited chemostat were analyzed in Northern blot, primer extension, and RT-PCR experiments.
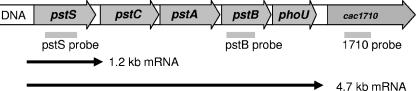
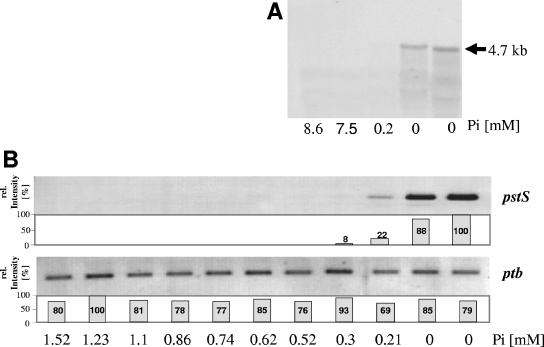
In Northern blots three different DIG-labeled DNA probes against pstS, pstB, and the open reading frame downstream of the phoU gene were used. Blots with the pstS probe clearly revealed two distinct mRNA signals, a dominating one of about 1.2 kb and a larger, less-abundant one of about 4.7 kb (schematically shown in Fig. 4; for original data, see Fig. 6, 7, and 8). No hybridization signal could be detected with the 1710 probe complementary to an internal fragment of the open reading frame cac1710 located downstream of phoU (not shown). The size of the larger 4.7-kb mRNA species represents the full pst operon including phoU (Fig. 4). The length of the small transcript (1.2 kb) is in agreement with the size of the pstS gene and was not detected with the pstB probe (see Fig. 6A).
FIG. 4.
Gene architecture and mRNA transcripts of the pst operon of C. acetobutylicum. Gray bars below the genes indicate the relative locations of probes used in Northern blot hybridizations. Arrows symbolize extension of the two detected mRNA species starting 80 nt upstream of pstS.
FIG. 6.
Induction of the pst operon of C. acetobutylicum under conditions of phosphate limitation. (A) Northern blot hybridization of total RNA (20 μg/lane) of cells from a chemostat culture (pH 4.5) after a Pi pulse (10 mM) with a DIG-labeled pstB probe. External phosphate concentrations are given below the lanes. (B) RT-PCR analyses of pstS and of the ptb gene (encoding phosphotransbutyrylase, constitutively expressed under the experimental conditions) as a positive control. Bars represent relative signal intensities. The maximal signal intensity was set as 100%.
FIG. 7.
Transcript levels of the pst operon of C. acetobutylicum are dependent on the pH. The pH in a phosphate-limited chemostat was increased from 4.5 to 5.8 at time 0. At different time points (2 h, 7 h, 30 h, 72 h) the levels of the products butanol (filled squares) and butyrate (open squares) were determined (A) and Northern blot analysis of total RNA (10 μg per lane) with a DIG-labeled pstS probe was performed (B).
FIG. 8.
Comparison of transcript levels of the pst operon in C. acetobutylicum cells grown at steady state in a phosphate-limited chemostat at pH 4.5 or pH 5.8. The different RNA amounts used for a semiquantitative Northern blot analysis are given in micrograms below the lanes. A pstS fragment was used as a probe.
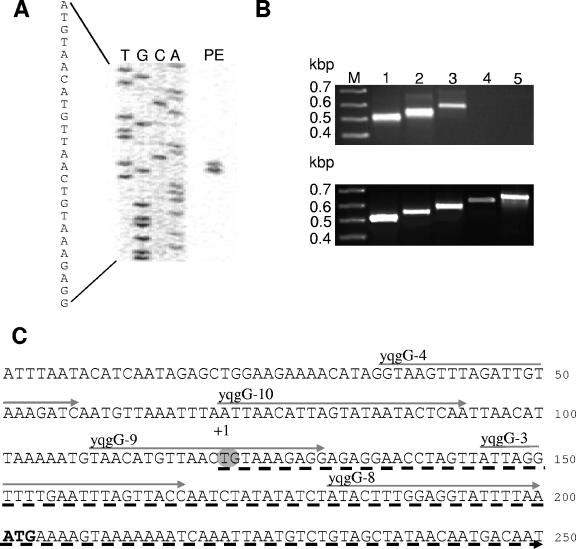
The transcription start point or more precisely the 5′ end of the mRNA of the pst operon was determined by primer extension analysis. Experiments with two independent, nonoverlapping DIG-labeled oligonucleotides (Table 1) identified an identical signal, which is shown in Fig. 5A. RT-PCR experiments confirmed the location of the transcription start site (Fig. 5B). Thereafter, reverse-transcribed pstS-specific cDNA served as template in PCRs in which five different “forward” primers were independently combined with one “reverse” primer. With the “forward” oligonucleotides located within the predicted mRNA transcripts strong PCR fragments could be generated, whereas only a faint signal was visible with the oligonucleotide yqgG-9 because only 10 bases were able to hybridize. No PCR products could be found with the “forward” primers located beyond the 5′ end of pst operon mRNA transcripts.
FIG. 5.
Mapping of the transcription start point of the pst operon of C. acetobutylicum. (A) The product of a primer extension reaction using IRD800-labeled oligonucleotide yqgG-PE-2 (PE) was run on a polyacrylamide gel alongside with the corresponding sequencing reactions (T, G, C, A) generated with the same primer. (B) RT-PCR products based on pstS-specific cDNA as the template (upper photo) compared with PCR products generated with plasmid DNA (pUM8) as positive controls (lower photo). DNA fragments were separated electrophoretically in a 1.5% (wt/vol) agarose gel and visualized by ethidium bromide staining. “Forward” primers (lanes 1 to 5, yqgG-8, yqgG-3, yqgG-9, yqgG-10, and yqgG-4; lanes M, marker) were separately combined with yqgG-2 as “reverse” primer. (C) Locations of “forward” primers within the DNA region spanning the transcription start point of the pstS operon (gray arrows above the DNA sequence) as well as the identified transcription start point (gray circle) are highlighted. The dashed arrow below the DNA sequence symbolizes mRNA and the bold ATG the start codon of pstS.
Thus, it can be concluded that transcription of the pst operon of C. acetobutylicum initiates 80 to 79 nucleotides (nt) upstream of the pstS gene at a T or G (Fig. 5C).
Transcription of the pst operon is restricted to phosphate limitation and is affected by the pH.
To define a threshold concentration of Pi for the expression of the pst operon, Pi pulses (10 mM) were applied into the phosphate-limited chemostat culture at steady state at pH 4.5 and 5.8. In the time period after the pulse, which was characterized by decreasing phosphate concentrations, total RNA samples were prepared until again no phosphate was detectable in the supernatant. Northern blots detected pst operon-specific signals only below an external Pi concentration of 0.2 mM, i.e., when phosphate was not measurable (Fig. 6A). These results were confirmed by more-sensitive RT-PCR experiments. Although basal amounts of RT-PCR products were visible around 0.2 mM Pi a strong signal was found again at 0 mM Pi (Fig. 6B). These data clearly suggest a threshold concentration for the expression of the pst operon in C. acetobutylicum of below 0.2 mM Pi.
Another interesting finding was that the transcript level of the pst operon is affected by the pH. As shown in Fig. 7, a shift of the pH in the culture vessel from 4.5 up to 5.8 (metabolic switch) increased the transcript level of the pst operon. Interestingly, the proportion of the smaller mRNA seemed to be more strongly increased than that of the full-length mRNA. Therefore, we analyzed the ratios of the transcripts in cells growing under steady-state conditions at pH 4.5 or pH 5.8 in more detail using semiquantitative Northern blot experiments (35). Serially diluted total RNA samples were hybridized against the pstS probe. Comparison of signal intensities clearly revealed amounts of the 4.7-kb transcript at least two times higher at pH 5.8 than at pH 4.5 (Fig. 8). The increase of the short pstS-specific mRNA amount was even more pronounced (four- to eightfold).
DISCUSSION
ABC importers of bacteria with their periplasmic binding protein are membrane transport systems, which are needed when the concentration of the specific substrate falls below a threshold concentration. For example, in E. coli the essential nutrient phosphate is taken up by the low-affinity Pit system as long as the concentration in the growth medium is above 4 μM (45). Below this level the high-affinity Pst system is induced. All components of this ABC transporter are encoded by the pstSCAB-phoU operon together with a protein called PhoU (46). Here we demonstrated that an identical operon exists in C. acetobutylicum which is induced under conditions of Pi limitation. In similarity to Pit results, a low-affinity phosphate uptake system, encoded by the open reading frame cac3093 and annotated as phosphate permease, is also present in C. acetobutylicum (14). However, the external Pi concentration that results in an expression of the pst-phoU operon in C. acetobutylicum (0.2 mM) was found to be 50-fold higher than that seen with E. coli. In this respect, the situation resembles the results for B. subtilis. There, the corresponding operon is induced at Pi concentrations below 0.1 mM (16, 17), whereas at higher phosphate concentrations the pit gene (ykaB) has been suggested to code for the low-affinity phosphate transporter under this condition (http://genolist.pasteur.fr/SubtiList). On the other hand, in the more closely related B. subtilis the operon structure is quite different due to the presence of two pstB genes and the absence of the phoU gene (37).
Our analyses demonstrate that transcription of the pst-phoU operon in C. acetobutylicum is initiated at one site and results in two mRNA molecules of different lengths (1.2 kb and 4.7 kb). The short transcript was found at significant higher levels and represents pstS-specific mRNA. Since a stem-loop structure of limited stability (47) is present 9 nt after the TAG stop codon of pstS (Fig. 9) we speculate that premature transcription termination downstream of pstS causes the generation of the two pst transcripts in different amounts. The proposed transcription terminator comprises a stem of 17 bp, including one imperfect U-G pairing and one C-A mismatch, as well as a bulb of 10 nt. The structure is followed by a uracil-rich sequence (UUUUAAUUUU). Thus, the sequence shows all basic characters of Rho-independent transcription terminators (10, 31, 34). On the other hand, the calculated free energy (51) of ΔG = −16.5 kJ mol−1 seems to be rather low and might explain the observed level of read-through. In the case of other putative Rho-independent termination sequences in C. acetobutylicum higher values were reported, i.e., for the one between the sol operon and the adc gene (ΔG = −75.2 kJ mol−1) or for the two hairpins following orf5 (solR) (ΔG = −90.9 kJ mol−1 and ΔG = −93.0 kJ mol−1) (13, 27).
FIG. 9.
A hairpin structure in the intergenic region between pstS and pstC. UAG represents the stop codon of pstS gene and AUG the start codon of pstC gene; the putative ribosome binding site of pstC is given in bold letters.
In E. coli, several transcripts of the pst-phoU operon have been observed by Northern blot analysis, including two pstS transcripts of different lengths (0.95 kb and 1.2 kb), but no full-length mRNA. Aguena et al. (1) supposed that a combination of at least two different mechanisms, premature termination and nucleolytic cleavage of the primary transcript, is responsible for this observation. Thus, the stable hairpin structure downstream of pstS in E. coli (ΔG = −72.8 kJ/mol) (22) not only causes partial termination but also stabilizes upstream mRNA by protecting it from exonucleolytic attack. The fact that in C. acetobutylicum only two pst transcripts, one pstS specific and one full length, could be detected led us to the conclusion that cleavage of pst mRNA might not occur in C. acetobutylicum. Thus, E. coli and C. acetobutylicum seem to have evolved different strategies to cope with the need for different amounts of the components of the phosphate-specific transporter which are encoded in one transcriptional unit. Generally, the abundance of pstS-specific mRNA in C. acetobutylicum is in good agreement with observations made with respect to other operons encoding ABC transporters. Usually, the first gene which encodes the periplasmic binding protein is transcribed in excess. Since binding of the substrate to the uptake system is predicted to be the rate-limiting step of the whole transport process, excess of the binding protein could facilitate an efficient transport (36).
One unexpected result of our investigation was the discovery that the transcript level of the pst operon of C. actobutylicum under conditions of phosphate limitation is also dependent on the pH. A switch from pH 4.5 to 5.8 generally increased the transcript level and further enhanced the dominance of the pstS transcript. It is not clear yet whether the observed higher transcript levels at pH 5.8 are due to a change in the transcription rate or in the stability of the mRNA. Interestingly, this observation is in good agreement with the idea of faster phosphate consumption of the cells under these conditions (see Fig. 1). In B. subtilis and B. licheniformis a sudden increase of the external pH induced transcription of the pst operon in cells growing under conditions of a surplus of Pi (5, 15). The alkaline pH mimics phosphate starvation by affecting a low-affinity phosphate uptake system. This can hardly explain the situation in C. acetobutylicum, as we used cells which still grew under conditions of phosphate limitation when the external pH was increased.
In other bacteria, the pst operon is a member of the so-called phosphate (Pho) regulon, under the control of a two-component regulatory system. Well-known examples are the sensor kinases-response regulators PhoR/PhoP in B. subtilis (16, 18) and PhoR/PhoB in E. coli (23, 46, 48). We have evidence that two genes further upstream of pstS encode such a two-component regulatory system for phosphate regulation in C. acetobutylicum (our unpublished results). Essential for the binding of the response regulator are conserved DNA motifs, so-called Pho boxes, replacing the −35 promoter elements. In B. subtilis, the Pho box consensus is represented by at least two repeats of an AT-rich TTTACA-like hexamer motif (16) which are separated by four to five bases (TT[A/T/C]ACA-N4-5-TT[A/T/C]ACA) (12, 21, 37). In E. coli Pho boxes are composed of two slightly modified heptamer sequences separated by four semiconserved nucleotides (CTGTCAT-A[A/T]A[T/A]-CTGT[C/A]A[C/T]). Interestingly, the pst promoter region in both organisms contains two consecutive Pho boxes (19, 37, 46). Analysis of the promoter region of the pst operon in C. acetobutylicum identified two overlapping −10 elements (Fig. 10), each matching the hexamer consensus motif (TATAAT) of vegetative clostridial promoters (49) in four positions. In contrast, in the −35 region no similarity to the consensus promoter is obvious. However, two identical 11-nt repeats span the −35 promoter region and are separated by another 11 nt (Fig. 10). The hexamer core motif of the B. subtilis Pho box (TT[A/T/C]ACA) is present nearly in the center of these direct repeats. At a distance of 5 nt, these motifs are preceded by a similar one in which two bases are changed. In summary, two putative clostridial Pho box-like sequences are present upstream of pstS, resembling the situations in B. subtilis and E. coli. A space of 11 bp between the 11-bp direct repeats may allow multiple binding of regulatory proteins at the same face of the DNA helix, as was well documented for PhoB of E. coli (8, 46). Another feature of this Pho box region in C. acetobutylicum has to be mentioned. At least eight bases of the first direct repeat are also part of an inverted repeat (Fig. 10). At the moment it can only be speculated upon whether this structure is, e.g., a binding site for an additional regulatory protein.
FIG. 10.
Putative regulatory sites in the promoter region of the pst operon of C. acetobutylicum. The transcription start point of the pst operon is marked by a gray circle and +1. The deduced −10 and −35 promoter sequences are boxed. Bold arrowheads above the sequence indicate direct 11-bp repeats and arrowheads below the sequence an inverted repeat. Thick bars over sequences represent those exactly matching the B. subtilis Pho box motif, and thin bars over sequences represent those of similar motifs in which two nucleotides are changed. The ribosome binding site of pstS is underlined, and the start codon is printed in bold letters.
The elucidation of the regulatory sites in the promoter region of the pst-phoU operon which integrate two different extracellular signals, depletion of Pi and the pH of the environment, will be an important task. The fact that under these conditions a metabolic switch occurs in C. acetobutylicum makes it even more interesting.
Acknowledgments
This work was supported, in part, by a grant from the Deutsche Forschungsgemeinschaft and fellowships of the Graduiertenförderung of the State of Mecklenburg-Vorpommern to M.M. and T.F.
REFERENCES
- 1.Aguena, M., E. Yagil, and B. Spira. 2002. Transcriptional analysis of the pst operon of Escherichia coli. Mol. Gen. Genet. 268:518-524. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ames, G. F. 1986. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu. Rev. Biochem. 55:397-425. [DOI] [PubMed] [Google Scholar]
- 4.Antelmann, H., C. Scharf, and M. Hecker. 2000. Phosphate starvation-inducible proteins of Bacillus subtilis: proteomics and transcriptional analysis. J. Bacteriol. 182:4478-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atalla, A., and W. Schumann. 2003. The pst operon of Bacillus subtilis is specifically induced by alkali stress. J. Bacteriol. 185:5019-5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahl, H., W. Andersch, and G. Gottschalk. 1983. 1982. Continuous production of acetone and butanol by Clostridium acetobutylicum in a two-stage phosphate limited chemostat. Eur. J. Appl. Microbiol. Biotechnol. 15:201-205. (Erratum, 17: 73). [Google Scholar]
- 7.Bieleski, R. L. 1973. Phosphate pools, phosphate transport, and phosphate availability. Annu. Rev. Plant Physiol. 24:225-252. [Google Scholar]
- 8.Blanco, A. G., M. Sola, F. X. Gomis-Rüth, and M. Coll. 2002. Tandem DNA recognition by PhoB, a two-component signal transduction transcriptional activator. Structure 10:701-713. [DOI] [PubMed] [Google Scholar]
- 9.Braibant, M., P. Lefevre, L. de Wit, P. Peirs, J. Ooms, K. Huygen, A. B. Andersen, and J. Content. 1996. A Mycobacterium tuberculosis gene cluster encoding proteins of a phosphate transporter homologous to the Escherichia coli Pst system. Gene 176:171-176. [DOI] [PubMed] [Google Scholar]
- 10.d'Aubenton Carafa, Y., E. Brody, and C. Thermes. 1990. Prediction of rho-independent Escherichia coli transcription terminators. A statistical analysis of their RNA stem-loop structures. J. Mol. Biol. 216:835-858. [DOI] [PubMed] [Google Scholar]
- 11.Dürre, P., and H. Bahl. 1996. Microbial production of acetone/butanol/isopropanol, p. 229-268. In P. Stadler (ed.), Biotechnology: a multi-volume comprehensive treatise, 2nd ed., vol. 1. VCH Verlagsgesellschaft, Weinheim, Germany. (In German.) [Google Scholar]
- 12.Eder, S., W. Liu, and F. M. Hulett. 1999. Mutational Analysis of the phoD promoter in Bacillus subtilis: implications for PhoP binding and promoter activation of Pho regulon promoters. J. Bacteriol. 181:2017-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer, R.-J., J. Helms, and P. Dürre. 1993. Cloning, sequencing, and molecular analysis of the sol operon of Clostridium acetobutylicum, a chromosomal locus involved in solventogenesis. J. Bacteriol. 175:6959-6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer, R.-J., and H. Bahl. 2005. Transport of phosphate, p. 287-294. In P. Dürre (ed.), Handbook on clostridia. CRC Press, Boca Raton, Fla.
- 15.Hornbaek, T., M. Jakobsen, J. Dynesen, and A. K. Nielsen. 2004. Global transcription profiles and intracellular pH regulation measured in Bacillus licheniformis upon external pH upshifts. Arch. Microbiol. 182:467-474. [DOI] [PubMed] [Google Scholar]
- 16.Hulett, F. M. 2002. The Pho regulon, p. 193-201. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and the closest relatives: from genes to cells. ASM Press, Washington, D.C.
- 17.Hulett, F. M. 1996. The signal-transduction network for pho regulation in Bacillus subtilis. Mol. Microbiol. 19:933-939. [DOI] [PubMed] [Google Scholar]
- 18.Hulett, F. M., J. Lee, L. Shi, G. Sun, R. Chesnut, E. Sharkova, M. F. Duggan, and N. Kapp. 1994. Sequential action of two-component genetic switches regulates the PHO regulon in Bacillus subtilis. J. Bacteriol. 176:1348-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura, S., K. Makino, H. Shinagawa, M. Amemura, and A. Nakata. 1989. Regulation of the phosphate regulon of Escherichia coli: characterization of the promoter of the pstS gene. Mol. Gen. Genet. 215:374-380. [DOI] [PubMed] [Google Scholar]
- 20.Kyte, J., and R. F. Doolittle. 1982. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157:105-132. [DOI] [PubMed] [Google Scholar]
- 21.Liu, W., and F. M. Hulett. 1997. Bacillus subtilis PhoP binds to the phoB tandem promoter exclusively within the phosphate starvation-inducible promoter. J. Bacteriol. 179:6302-6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magota, K., N. Otsuji, T. Miki, T. Horiuchi, S. Tsunasawa, J. Kondo, F. Sakiyama, M. Amemura, T. Morita, H. Shinagawa, and A. Nakata. 1984. Nucleotide sequence of the phoS gene, the structural gene for the phosphate-binding protein of Escherichia coli. J. Bacteriol. 157:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makino, K., H. Shinagawa, M. Amemura, S. Kimura, A. Nakata, and A. Ishihama. 1988. Regulation of the phosphate regulon of Escherichia coli. Activation of pstS transcription by PhoB protein in vitro. J. Mol. Biol. 203:85-95. [DOI] [PubMed] [Google Scholar]
- 24.May, A., F. Hillmann, O. Riebe, R.-J. Fischer, and H. Bahl. 2004. A rubrerythrin-like oxidative stress protein of Clostridium acetobutylicum is encoded by a duplicated gene and identical to the heat shock protein Hsp21. FEMS Microbiol. Lett. 238:249-254. [DOI] [PubMed] [Google Scholar]
- 25.Meinecke, B., H. Bahl, and G. Gottschalk. 1984. Selection of an asporogenous strain of Clostridium acetobutylicum in continuous culture under phosphate limitation. Appl. Environ. Microbiol. 48:1064-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell, W. J. 2001. Spores and sporulation, p. 72-83. In P. Dürre (ed.), Clostridia. Biotechnology and medical applications. Wiley-VCH Verlag GmbH, Weinheim, Germany. (In German.)
- 27.Nair, R. V., E. M. Green, D. E. Watson, G. N. Bennett, and E. T. Papoutsakis. 1999. Regulation of the sol locus genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 by a putative transcriptional repressor. J. Bacteriol. 181:319-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikata, T., Y. Sakai, K. Shibat, J. Kato, A. Kuroda, and H. Ohtake. 1996. Molecular analysis of the phosphate-specific transport (pst) operon of Pseudomonas aeruginosa. Mol. Gen. Genet. 250:692-698. [DOI] [PubMed] [Google Scholar]
- 29.Nölling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. Zeng, R. Gibson, H. Mei Lee, J. Dubios, D. Qiu, J. Hitti, GTC Sequencing Center Production, Finishing, and Bioinformatics Teams, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novak, R., A. Cauwels, E. Charpentier, and E. Tuomanen. 1999. Identification of a Streptococcus pneumoniae gene locus encoding proteins of an ABC phosphate transporter and a two-component regulatory system. J. Bacteriol. 181:1126-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nudler, E., and M. E. Gottesman. 2002. Transcription termination and anti-termination in E. coli. Genes Cells 7:755-768. [DOI] [PubMed] [Google Scholar]
- 32.Oelmüller, U., N. Krüger, A. Steinbüchel, and C. G. Freidrich. 2002. Isolation of prokaryotic RNA and detection of specific mRNA with biotinylated probes. J. Microbiol. Methods 11:73-81. [Google Scholar]
- 33.Oultram, J. D., M. Loughlin, T.-J. Swinfield, J. K. Brehm, D. E. Thompson, and N. P. Minton. 1988. Introduction of plasmids into whole cells of Clostridium acetobutylicum by electroporation. FEMS Microbiol. Lett. 56:83-88. [Google Scholar]
- 34.Platt, T. 1986. Transcription termination and the regulation of gene expression. Annu. Rev. Biochem. 55:339-372. [DOI] [PubMed] [Google Scholar]
- 35.Podbielski, A., A. Flosdorff, and J. Weber-Heynemann. 1995. The group A streptococcal virR49 gene controls expression of four structural vir regulon genes. Infect. Immun. 63:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Podbielski, A., B. Pohl, M. Woischnik, C. Korner, K.-H. Schmidt, E. Rozdzinski, and B. A. B. Leonard. 1996. Molecular characterization of group A streptococcal (GAS) oligopeptide permease (Opp) and its effect on cysteine protease production. Mol. Microbiol. 21:1087-1099. [DOI] [PubMed] [Google Scholar]
- 37.Qi, Y., Y. Kobayashi, and F. M. Hulett. 1997. The pst operon of Bacillus subtilis has a phosphate-regulated promoter and is involved in phosphate transport but not in regulation of the Pho regulon. J. Bacteriol. 179:2534-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao, N. N., and A. Torriani. 1990. Molecular aspects of phosphate transport in Escherichia coli. Mol. Microbiol. 4:1083-1090. [DOI] [PubMed] [Google Scholar]
- 39.Rogers, P. G., and G. Gottschalk. 1993. Biochemistry and regulation of acid and solvent formation in clostridia, p. 25-50. In D. R. Woods (ed.), The clostridia and biotechnology. Butterworth-Heinemann, London, United Kingdom.
- 40.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- 41.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steed, P. M., and B. L. Wanner. 1993. Use of the rep technique for allele replacement to construct mutants with deletions of the pstSCAB-phoU operon: evidence of a new role for the PhoU protein in the phosphate regulon. J. Bacteriol. 175:6797-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takemaru, K., M. Mizuno, and Y. Kobayashi. 1996. A Bacillus subtilis gene cluster similar to the Escherichia coli phosphate-specific transport (pst) operon: evidence for a tandemly arranged pstB gene. Microbiology 142:2017-2020. [DOI] [PubMed] [Google Scholar]
- 44.Thormann, K., L. Feustel, K. Lorenz, S. Nakotte, and P. Dürre. 2002. Control of butanol formation in Clostridium acetobutylicum by transcriptional activation. J. Bacteriol. 184:1966-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wanner, B. L. 1993. Gene regulation by phosphate in enteric bacteria. J. Cell. Biochem. 51:47-54. [DOI] [PubMed] [Google Scholar]
- 46.Wanner, B. L. 1996. Phosphorus assimilation and control of the phosphate regulon, p. 1357-1381. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington D.C.
- 47.Wilson, K. S., and P. H. von Hippel. 1995. Transcription termination at intrinsic terminators: the role of the RNA hairpin. Proc. Natl. Acad. Sci. USA 92:8793-8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada, M., K. Makino, M. Amemura, H. Shinagawa, and A. Nakata. 1989. Regulation of the phosphate regulon of Escherichia coli: analysis of mutant phoB and phoR genes causing different phenotypes. J. Bacteriol. 171:5601-5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young, M., N. P. Minton, and W. L. Staudenbauer. 1989. Recent advances in the genetics of the clostridia. FEMS Microbiol. Rev. 63:301-326. [DOI] [PubMed] [Google Scholar]
- 50.Zilversmit, D. B., and A. K. Davis. 1950. Microdetermination of plasma phospholipids by trichloroacetic acid precipitation. J. Lab. Clin. Med. 35:155-160. [PubMed] [Google Scholar]
- 51.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]