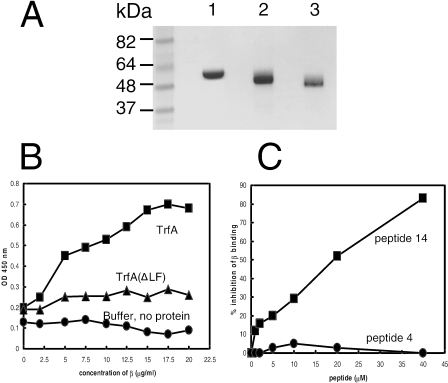
FIG. 2.
(A) SDS-PAGE analysis of purified wild-type β2 (lane 1), His-tagged TrfA-33 (lane 2), and His-tagged TrfA-33 (ΔLF) (lane 3) proteins. Three to five micrograms of each purified protein was electrophoresed through a 4 to 12% SDS-PAGE gel prior to being stained with Coomassie brilliant blue R-250 (Bio-Rad). Positions of size standards are indicated. (B) Interactions of β2 with TrfA-33 proteins. Increasing concentrations of β2 protein were added to TrfA-33 proteins immobilized onto microtiter plates (5 μg/ml, 50 μl/well). Bound β2 proteins were detected with anti-β2 antibody. ▪, ΤrfA-33 on plate; ▴, TrfA-33 (ΔLF) on plate; •, binding buffer, no protein on plate. (C) β2-binding consensus peptide, peptide 14 with the sequence IG QLSLF GV, inhibited the interaction of TrfA-33 and β2. Microtiter plate assay results showing the inhibition of TrfA-33 and β2 interaction by addition of various amounts of peptide 14. No inhibition was observed with the control peptide, peptide 4 with the sequence IG QADMA GV. ▪, peptide 14; •, peptide 4.