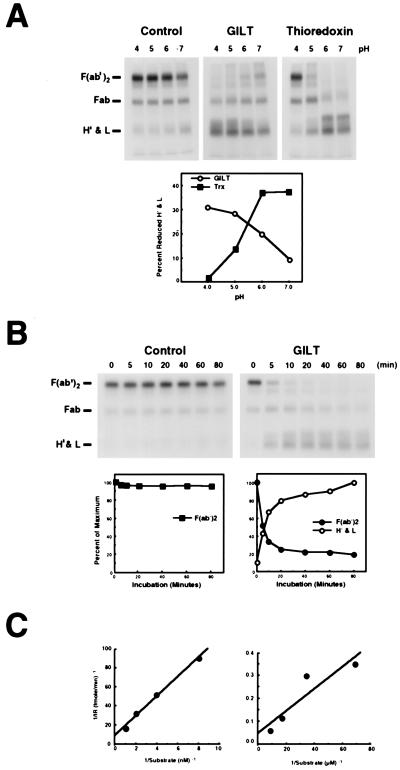
Figure 3.
GILT is an acid optimal thiol reductase. (A) Lysozyme (negative control), GILT (550 nM), or thioredoxin (1.04 μM) was incubated with 125I-F(ab′)2 (55 nM) under different pH conditions for 45 min at 37°C. (B) Lysozyme or GILT (550 nM) was incubated with 125I-F(ab′)2 (55 nM) at pH 4.0 and 37°C, and the reaction was stopped with excess iodoacetamide at different periods of time. (C) To quantitate the effect of substrate denaturation on GILT activity, 125 pM to 1 nM SDS-denatured 125I-F(ab′)2 (Left) and 14.5 nM to 116 nM native 125I-F(ab′)2 (Right) were incubated for 10 min at 37°C with 69 nM or 1.1 μM GILT, respectively, stopping the reaction by the addition of iodoacetamide. Samples were separated by nonreducing SDS/PAGE (12%) and visualized by autoradiography. In A and B, the gels are shown, and the positions of F(ab′)2, Fab, H′ and light chains are indicated (Left). The intensity of specific bands (pixel density) was quantitated and plotted in A as percent reduced heavy and light chains after background subtraction and in B as percent of maximum. In C, the bands were converted to molarity of substrate. The results are presented as Lineweaver–Burke plots.