Figure 3. Hsp104 catalyzes or reverses the formation of Sup35 oligomers that nucleate prion assembly.
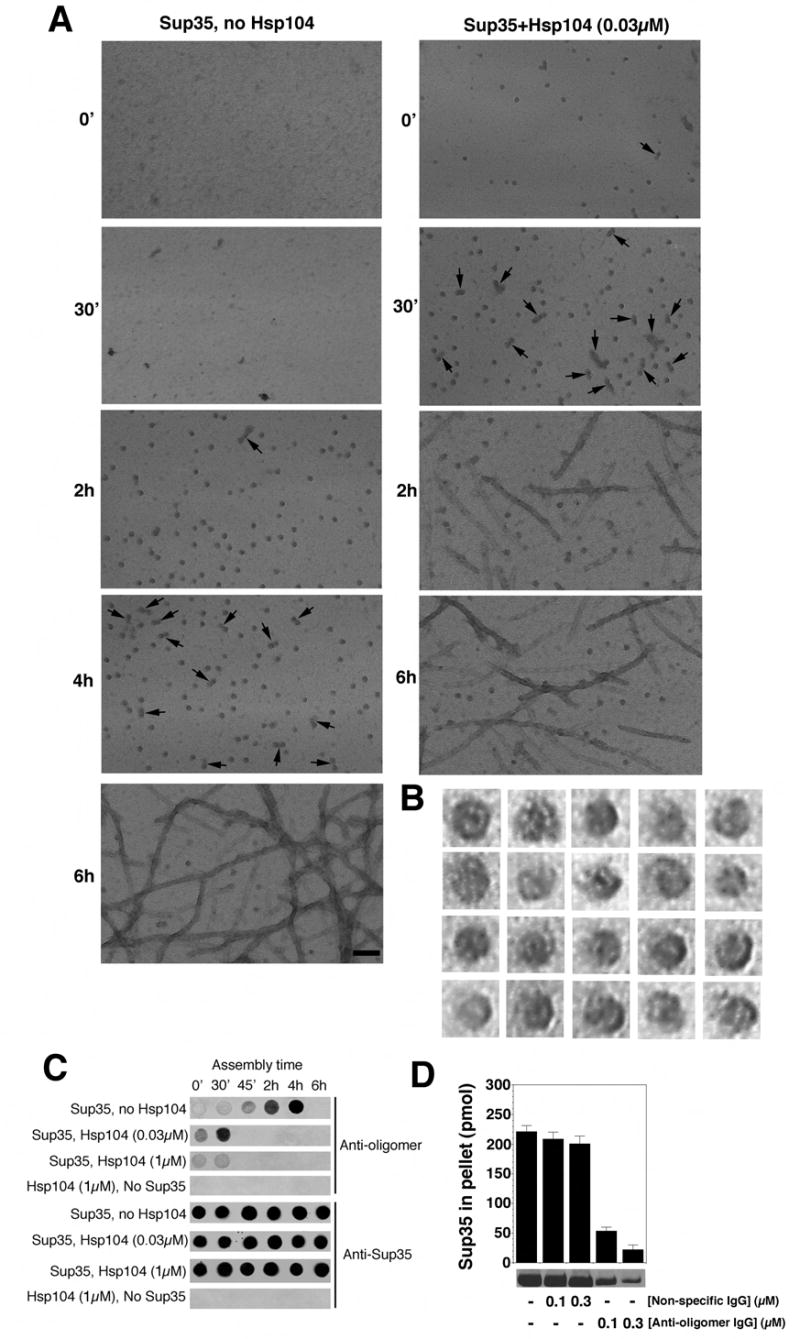
(A) Unseeded, rotated (80rpm) Sup35 (2.5μM) fibrillization reactions were performed without or with Hsp104 (0.03μM) plus ATP (5mM). At various times, reactions were processed for EM. Arrows denote close appositions of spherical oligomers. These oligomers assembled with Hsp104 did not correspond to Hsp104 because they were not observed in the absence of Sup35 (not shown). Although Hsp104 was hexameric in our conditions (not shown), visualization of Hsp104 hexamers by negative stain typically requires higher concentrations of Hsp104 and fixation with glutaraldehyde (Parsell et al., 1994a). Bar, 0.1μm.
(B) Gallery of twenty separate Sup35 oligomers formed after 2h without Hsp104. EM images are 29nm squares.
(C) Unseeded, rotated (80rpm) Sup35 (2.5μM) fibrillization reactions were performed without or with Hsp104 (0.03μM or 1μM) plus ATP (5mM). At various times, reactions were applied to nitrocellulose and probed with anti-oligomer antibody or anti-Sup35 antibody. Reactions lacking Sup35 were also assessed.
(D) Unseeded, rotated (80rpm) Sup35 (2.5μM) fibrillization reactions were performed without or with non-specific IgG (0.1–0.3μM) or anti-oligomer IgG (0.1–0.3μM). After 8h reactions were processed for sedimentation analysis. Values represent means±SD (n=3).
