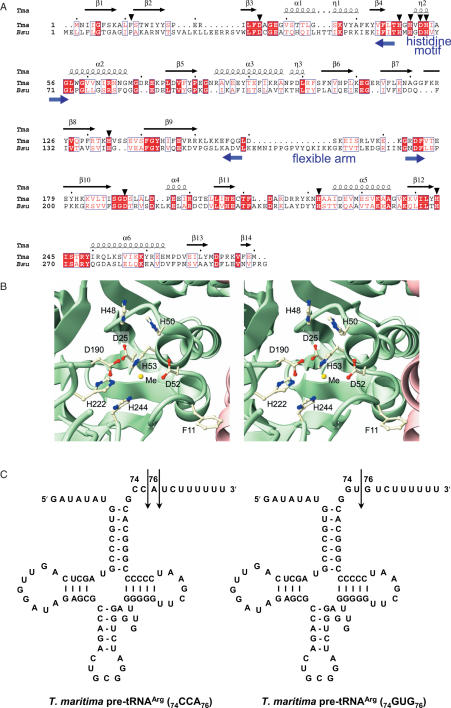
Figure 1.
Structures of T.maritima tRNase Z and pre-tRNAs. (A) The amino acid sequences of T.maritima (Tma) and B.subtilis (Bsu) tRNase Zs are aligned using ClustalW (25) and represented using ESPript (26). The T.maritima tRNase Z secondary structures for α-helices, β-strands and 310 helices are indicated by α, β and η, respectively. The red box and the blue rectangle denote the conserved and moderately conserved residues, respectively. The regions of the histidine motif, the flexible arm and the mutated amino acid residues (triangle) are shown. (B) Stereoview for the catalytic center of T.maritima tRNase Z (14). The two subunits are colored green and pink, respectively. The mutated residues, except His-134, which is disordered, are represented by ball-and-stick models. One unidentified metal ion is depicted as a yellow sphere. (C) T.maritima pre-tRNAArg(74CCA76) and pre-tRNAArg(74GUG76). The sequence 74CCA76 in the former is substituted with 74GUG76 in the latter. Cleavage sites are denoted by arrows.