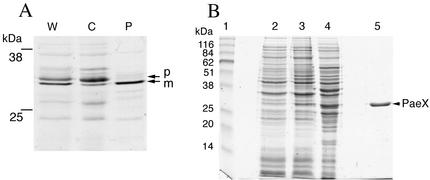
FIG. 4.
Fractionation and purification of the PaeX protein. (A) Cellular localization of PaeX in E. coli. Cells of E. coli K38/pGP1.2/pN2170 were labeled with [35S]cysteine-methionine. The periplasmic proteins were extracted from the labeled cells by osmotic shock. W, whole cells; P, periplasmic fraction; C, osmotically shocked cell fraction. The proteins were separated by SDS-PAGE, and the gels were autoradiographed. Precursor (p) and mature (m) forms of PaeX are indicated. (B) Purification of PaeX. Shown are whole-cell lysates of E. coli BL21(DE3)/pN2170 before (lane 2) and after (lane 3) induction, extract from induced cells (lane 4), and purified PaeX (lane 5). The proteins were separated by SDS-PAGE and stained with Coomassie blue G-250. Apparent molecular masses of the standards (lane 1) are indicated. The position of PaeX is indicated.