Abstract
This study describes a novel helicase-mediated isothermal DNA amplification method that exponentially amplifies circular DNAs. The circular helicase-dependent amplification (cHDA) system is based on the T7 replication machinery, which includes the processive T7 helicase, an exonuclease-deficient T7 DNA polymerase (T7 Sequenase) and the T7 Gp2.5 single-stranded DNA-binding (SSB) protein. After the duplex DNA template is unwound by the T7 helicase, specific primers anneal to the separated DNA strands and T7 Sequenase extends the 3′ end of each primer by a rolling circle mechanism to amplify not only a region defined by the primers but also continuous concatemers of the template. The cHDA reaction can be carried out at one temperature (25°C) for the entire process and can achieve up to 10 000-fold amplification. Amplification can be performed using purified plasmid DNA or a crude cell lysate and can amplify inserts as large as 10 kb. Following a cHDA reaction, the amplified products can be used directly for sequencing and restriction enzyme digestion without further purification. By utilizing the helicase enzyme, circular DNA samples can be simultaneously screened and amplified at one constant temperature in one easy step.
INTRODUCTION
DNA amplification is a basic technique and an essential tool in biomedical research and diagnostics. The most widely used method, the polymerase chain reaction (PCR), amplifies specific DNAs, delimited by two complementary oligonucleotide primers, using a thermostable DNA polymerase (1). In PCR, temperature cycling mediates strand separation, primer annealing and primer extension, to produce two replicates of the template that are used in the next round of amplification.
Despite its widespread appeal, the need for temperature cycling in PCR limits its portability due to the significant energy used in such sensitive instrumentation. As a result, several isothermal DNA amplification methods have been developed. Strand displacement amplification (SDA) utilizes restriction endonucleases to nick double-stranded (ds) DNAs that are extended by nuclease-deficient DNA polymerases. The newly synthesized DNA is displaced to serve as a template for antisense DNA synthesis, resulting in exponential amplification of the target DNA (2). Restriction endonucleases complicate SDA reactions by necessitating the use of modified deoxynucleotides and primers. Similarly, multiply primed rolling circle amplification (RCA) is a complex method for amplifying circular DNAs using random hexamers and Φ29 DNA polymerase (3). Multiply primed RCA can achieve up to 10 000-fold amplification; however, the use of random primers leaves the technique sensitive to contaminating DNAs. Although SDA and RCA are described as isothermal amplification systems, both methods require an initial heat denaturation step.
Recently, a true isothermal DNA amplification technology that incorporates a helicase enzyme into the reaction scheme was reported (4). Helicase-dependent amplification (HDA) exploits the unwinding activity of a helicase to separate duplex DNA targets during in vitro DNA amplification, eliminating the need for thermocycling (4). Accessory proteins, like single-stranded DNA-binding (SSB) protein, assist the helicase by preventing reformation of dsDNA. Once the template DNA is separated, specific primers anneal and are extended by an exonuclease-deficient DNA polymerase to yield two replicates of the original DNA; thus, achieving exponential amplification. The first HDA system reported used the Escherichia coli UvrD helicase and was only able to amplify DNA fragments up to several hundred base pairs in size (4). It was suggested that the inefficiency of the UvrD–HDA system at amplifying long target sequences is due to the limited speed (20 bp/s) and processivity (100 bp per binding) of the UvrD helicase.
In this report, we describe a new helicase-based DNA amplification system that employs a highly processive helicase and selectively uses circular DNA molecules to amplify a very long DNA fragment. The circular helicase-dependent amplification (cHDA) system amplifies circular DNAs using the T7 bacteriophage replication machinery: T7 DNA polymerase (T7 gene 5 protein plus E.coli thioredoxin), T7 helicase (T7 gene 4B protein) and T7 SSB protein (gene 2.5 protein or gp2.5) (5,6). The T7 DNA polymerase is a non-processive polymerase; however, when it forms a complex with E.coli thioredoxin, DNA synthesis is catalyzed at a speed of >100 nt/s with a processivity >10 kb per binding event (7). The T7 DNA polymerase used in cHDA is T7 Sequenase (USB, Cleveland, OH), which lacks the 3′–5′ exonuclease activity and possesses strand displacement activity (8). The T7 gene 4 encodes two forms of the helicase, a 63 kDa (gene 4A) and a 56 kDa (gene 4B) protein (9). The translation of the 56 kDa protein is initiated at an internal initiation codon (Met64) that is in frame with the coding sequence of gene 4A. The T7 4A protein displays both helicase and primase activities (10); whereas, the T7 4B protein only exhibits a 5′–3′ helicase activity (11–15). Either form is highly processive, traveling an average of 75 kb of single-stranded DNA (ssDNA) before dissociating (16). The T7 gene 2.5 encodes a SSB protein that is able to bind ∼7 nt per monomer (17,18). The Gp2.5 protein stimulates DNA polymerase activity and increases the efficiency of RNA primer synthesis by physically interacting with the T7 DNA polymerase and T7 gene 4 helicase (19–21).
In cHDA, a modified T7 replisome amplifies circular DNA templates in vitro. Amplification requires two specific primers and a complete replisome, including T7 Sequenase, T7 helicase and T7 SSB. The cHDA platform can amplify not only the entire circular DNA template, but also a specific target sequence defined by two primers and, thus, plasmid amplification and screening can be simultaneously performed.
MATERIALS AND METHODS
Reagents
T7 Sequenase version 2.0 was purchased from USB Corporation (Cleveland, OH). Source 15Q and Heparin TSK resins for FPLC (fast protein liquid chromatography) were purchased from Amersham. Chitin resin, Taq DNA polymerase, restriction enzymes, T4 SSB, E.coli strains, nucleotides, DNA markers, primers and plasmids, were obtained from New England Biolabs (Beverly, MA).
Cloning and expression of T7 gene 4B helicase and gene 2.5 SSB proteins
Genes encoding T7 4B helicase (GenBank accession no. AAP33931) and T7 Gp2.5 SSB (GenBank accession no. AAP33924) were cloned using the Impact™ system (NEB). T7 gene 4B was amplified from T7 genomic DNA (NEB) using primer 4B51 (5′-ACCCTTTCATATGACTTACAACGTGTGGAACTTC-3′) and 4B31 (5′-GGAAATGCTCTTCCGCAGAAGTCAGTGTCGTTGGACC-3′). T7 gp2.5 was amplified from T7 genomic DNA using primer 2551(5′-ACCCTTTCATATGGCTAAGAAGATTTTCACCTCTGCGCTGGGTACCGCTGAACCTTACGCTTACATCGCCAAGCCGGACTACGGCAACGAGGAGCGTGGCTTTGGGA-3′) and 2531 (5′-GGAAATGCTCTTCCGCAGAAGTCTCCGTCTTCGTCTG-3′). PCR products were digested with SapI and NdeI and ligated into plasmid pTYB1 (NEB) to give pTYB4B or pTYB25 for expressing the T7 4B helicase and Gp2.5 SSB protein, respectively. The pTYB1 vector produces a protein that is fused to an intein/chitin binding domain tag for purification. Plasmid inserts were sequenced and confirmed to be free of PCR-generated mutations. The plasmid DNA was used to transform E.coli strain ER2566, which expresses the T7 RNA polymerase for heterologous protein expression (NEB). ER2566 cells containing either pTYB4B or pTYB25 were grown at 37°C in LuriaBertani (LB) media supplemented with 100 μg/ml ampicillin. At a cell density of OD600 = 0.65–0.8, protein expression was induced with 0.3 mM isopropyl-beta-d-thiogalactopyranoside (IPTG) and cells were incubated for 16 h at 15°C. Induced cells were harvested by centrifugation and pellets were stored at −20°C.
Purification of T7 helicase and SSB proteins
All purification procedures were performed at 4°C. For both the gene 4B and Gp2.5 proteins, cells from a 3 litre culture were resuspended in 60 ml column buffer (20 mM Tris–HCl, pH 8.0, 1 mM EDTA and 500 mM NaCl) and sonicated. Cell lysates were centrifuged at 12 000 g for 30 min and the clarified extract was applied to a column packed with 20 ml of chitin bead resin that had been equilibrated with column buffer. The column bed was then washed with 10 bed volumes of column buffer and self-cleavage was conducted by flushing the column with 3 bed volumes of cleavage buffer (20 mM Tris–HCl, pH 8.0, 1 mM EDTA, 500 mM NaCl and 50 mM DTT). The cleavage reaction was allowed to proceed at 4°C for 64 h and the protein was eluted with column buffer. The eluted protein was diluted to a final salt concentration of 50 mM NaCl and applied to 1 ml Source 15Q and then Heparin TSK columns using an FPLC. Purified gene 4B helicase protein was dialyzed against a storage buffer (20 mM Tris–HCl, pH 7.5, 100 mM NaCl, 0.1 mM EDTA, 1 mM DTT and 50% glycerol). Purified Gp2.5 SSB protein was dialyzed against a storage buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.1 mM EDTA, 1 mM DTT and 50% glycerol). Proteins were stored at −70°C.
cHDA reactions
Three plasmids were used as a template in this study: plasmid pREP is a pCR2.1-TOPO (Invitrogen, Carlsbad, CA) vector containing the E.coli Rep gene (2.2 kb) as an insert (GenBank accession no. U00096); plasmid pTopo10k is a pCR2. 1-TOPO vector containing a 10 kb DNA insert (Dr J. Foster, NEB, personal communication); pCR-4E is a PCR-BluntII-TOPO (Invitrogen) vector containing a 1 kb DNA insert and carries a unique BbvCIB site. Primers used in the study are listed in Table 1. A 50 μl cHDA reaction contained 1 U T7 Sequenase (USB), 1–1.5 μg T7 4B helicase, 8 μg T7 Gp2.5, plasmid DNA, 0.4 μM forward primer, 0.4 μM reverse primer, 0.6 mM dNTPs and 10 mM dTTP in 1× cHDA reaction Buffer (35 mM Tris–acetate, pH 7.5, 11 mM magnesium acetate, 5 mM DTT and 0.01% Triton X-100). The reactions were incubated at 25°C for 6 h and 10 μl was resolved through a 1% agarose gel. For a two-step cHDA reaction, templates and primers were denatured at 95°C for 3 min and cooled to 25°C, before they were added to the remaining cHDA components and incubated at 25°C for 6 h.
Table 1.
List of primers used in the cHDA reactions
| Primer (NEB) | Sequence |
|---|---|
| S1201 | 5′-d(AACAGCTATGACCATG)-3′ |
| S1226 | 5′-d(CATACGATTTAGGTGACACTATAG)-3′ |
| S1233 | 5′-d(AGCGGATAACAATTTCACACAGGA)-3′ |
| S1249 | 5′-d(ATTTAGGTGACACTATAG)-3′ |
| S1224 | 5′-d(CGCCAGGGTTTTCCCAGTCACGAC)-3′ |
| S1248 | 5′-d(TAATACGACTCACTATAGGG)-3′ |
| S1212 | 5′-d(GTTTTCCCAGTCACGAC)-3′ |
cHDA reactions from a colony
Plasmid pREP was used to transform E.coli ER2502 [NEB; fhuA2 ara-14 leu D(gpt-proA)62 lacY1 glnV44 galK2 rpsL20 endA1 R(zgb210::Tn10)Tet S xyl-5 mtl-1 D(mcrC-mrr)HB101]. Bacterial cells from a colony were resuspended in a cHDA reaction mix missing the three T7 proteins and incubated at 95°C for 3 min and cooled to 25°C, before the T7 proteins were added and the reaction was incubated at 25°C for 6 h.
Pulse-field gel electrophoresis
A 1% low melting agarose gel (Seakem LE agarose; Cambrex) was used in a contour clamped homogenous electric field at 6 V/cm. Switch times ramped from 1.5 to 11 s. The gel was run at 14°C for 16.5 h and Mid-Range I PFG marker and Low Range PFG Marker (NEB) were used as molecular weight standards.
RESULTS
Assembly of a T7 replisome-based HDA system: cHDA
A new HDA system based on the T7 replisome, named cHDA, was developed to overcome the limitation on the size of DNA fragments amplified by the UvrD-based HDA (4). The UvrD-based HDA system uses the E.coli UvrD helicase, Klenow Fragment (3′→ 5′ exo-) of DNA polymerase I and T4 Gp32 SSB, and it amplifies DNA fragments only up to several hundreds of base pairs. In order to amplify longer DNA fragments, the cHDA system employs fast and highly processive counterparts of the enzymes used by these authors, the T7 4B helicase and T7 Sequenase. In addition, the T4 Gp32 SSB used by Vincent et al. (4) was substituted by T7 Gp2.5 in the cHDA system.
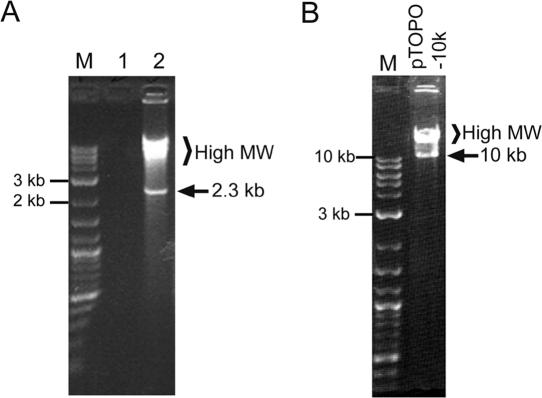
The performance of the cHDA system was examined using the pREP plasmid as a template and a pair of primers, S1233 and S1224 (Table 1), to amplify a 2.3 kb DNA fragment carrying the E.coli Rep gene. The cHDA reactions of pREP amplified a 2.3 kb DNA product as anticipated (Figure 1A, lane 2). Surprisingly, the reaction additionally generated multiple products with a higher molecular weight, but no product was observed in the absence of DNA template (Figure 1A, lane 1). The 2.3 kb DNA product corresponds to the size of the predicted product defined by the primers used, and sequencing results confirmed that the product was the Rep gene (data not shown).
Figure 1.
DNA amplification by the cHDA system. The cHDA reactions were performed according to Materials and Methods except that the reactions were incubated overnight at 25°C and that 5 μl of each reaction was then separated by gel electrophoresis in a 1% agarose gel. (A) The cHDA reactions were carried out in the absence (lane 1) or presence (lane 2) of pREP (6 kb; 10 ng) as a template. (B) The pTOPO-10k plasmid DNA (14 kb) was used as a template in the cHDA reaction. Lane M, 2-Log DNA ladder (NEB).
To determine the ability of the cHDA system to amplify longer DNA fragments, pTopo-10k, a vector containing a 10 kb insert flanked by primers S1233 and S1224, was used as a template in a cHDA reaction. Amplification of the pTopo10k plasmid by cHDA yielded the expected 10 kb product and higher molecular weight products, similar to those observed with the smaller pREP plasmid (Figure 1B).
Characterization of cHDA reaction products
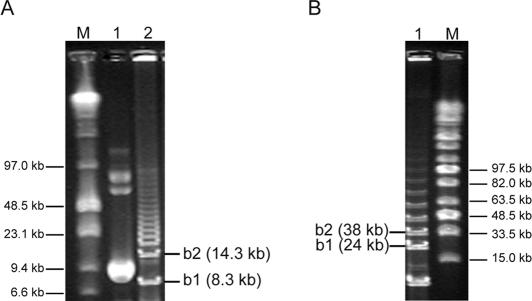
Amplification of plasmids by cHDA yielded a specific product, and a series of higher molecular weight products. To determine the identity of the higher molecular weight products, the amplification products of cHDA reactions using pREP or pTopo-10k as a template were analyzed by pulse-field gel electrophoresis. The cHDA products >2.3 or 10 kb specific products of the pREP or pTopo-10k reactions, respectively, were resolved into a ladder of DNA fragments (Figure 2). The smallest band, b1, of each ladder correlated with the predicted size of the entire plasmid and the insert (for pREP, 6 kb + 2.3 kb; for pTopo-10k, 14 kb + 10 kb), yielding an 8.3 kb band for pREP and a 24 kb band for pTopo-10k. The next band, b2, also correlates with the predicted size of two repeats of the plasmid and each respective insert. These observations suggest that the reaction mechanism of cHDA is by rolling circle and that the identity of the higher molecular weight products is a series of concatemers of the plasmid template (see Discussion).
Figure 2.
Separation of cHDA high molecular weight products. Products of cHDA were separated by pulse-field gel electrophoresis through a 1% low melt agarose gel. The size of the DNA markers are indicated. Two DNA bands located immediately above the specific products are shown as b1 and b2. (A) Pulse-field gel electrophoresis of cHDA reactions performed with the pREP plasmid (6 kb). Lane M, low range PFG marker (NEB); lane 1, pREP plasmid; lane 2, the cHDA product. (B) Pulse-field gel electrophoresis of cHDA reactions performed with the pTopo-10k plasmid (14 kb). Lane M, Mid-range PFG marker (NEB); lane 1, the cHDA products are as follows: lane 1, the pTopo-10k cHDA product and, lane M, mid-range PFG marker (NEB).
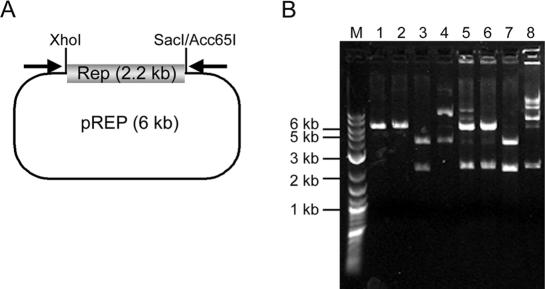
Restriction endonuclease digestions of the amplification products were performed to further analyze the nature of the concatemers produced in cHDA reactions. Acc65I, SacI and XhoI have a unique site within pREP (Figure 3A) and digestion with either Acc65I or SacI linearizes pREP, producing a 6 kb fragment (Figure 3B, lanes 1 and 2). When pREP was digested with XhoI and SacI, 2.2 and 3.8 kb fragments were detected (Figure 3B, lane 3). When the amplification product was digested with either Acc65I or SacI, the specific 2.3 kb insert was observed in addition to the linear 6 kb plasmid (Figure 5B lanes 5 and 6). When the amplification product was digested with XhoI and SacI, 3.8 and 2.2 kb fragments were produced, indicating that the 2.3 and 6 kb fragments produced by a single SacI digestion were further cleaved into 2.2 and 3.8 kb fragments by XhoI (Figure 3B, lane 7). The digestion results are consistent with our hypothesis that double-stranded concatemers with a unit repeat size of the plasmid were produced by the cHDA reaction and the smallest fragment is the specific target sequence defined by the two primers. This result also indicates that, after the plasmid amplification by cHDA, the products can be directly analyzed by restriction enzyme digestion.
Figure 3.
Restriction enzyme digestion of cHDA products. (A) pREP was created by inserting the 2.2 kb DNA fragment carrying the E.coli rep gene into the pCR2.1 TOPO vector. The 2.2 kb insert is flanked by XhoI and Sac I/Acc65I sites. Arrows indicate the locations of primers S1233 and S1224. (B) The pREP plasmid (lane 4) was digested with Acc65I (lane 1), SacI (lane 2), or SacI and XhoI (lane 3). The amplification products (lane 8) of a cHDA reaction using pREP as a template was digested with Acc65I (lane 5), SacI (lane 6), or SacI and XhoI (lane 7). The sizes of the DNA markers (lane M) are shown on the left.
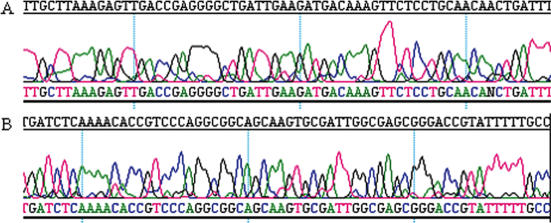
The use of cHDA reaction products for molecular biological purposes was further examined. The cHDA products were used in sequencing reactions using the forward primer S1233 (Figure 4A) or a Rep specific primer (Figure 4B). Sequencing reactions were performed using an ABI Sequencer and 1 μl of the cHDA product, without a post-amplification DNA purification step. The reactions yielded >600 bp of sequencing data with low background, indicating that the cHDA products can be directly used as sequencing templates without a purification step.
Figure 4.
Sequencing of cHDA products. (A) Sequencing with the forward primer of the cHDA reaction, S1233. (B) Sequencing with a Rep specific primer.
Analysis of the cHDA reaction components
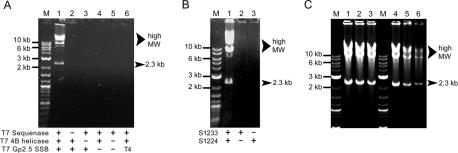
Amplification by cHDA yields a specific product defined by two primers and concatemers of the plasmid template. A series of cHDA reactions were designed to determine whether each reaction component is necessary for efficient amplification. Components of the T7 replisome required for cHDA were determined by omitting the polymerase, helicase or SSB protein from the reaction. A complete reaction containing the T7 Sequenase, helicase and SSB proteins supported efficient product formation (Figure 5A, lane 1). In the absence of T7 Sequenase, helicase or SSB protein, no amplification was observed (Figure 5A, lanes 2–4), confirming that each protein is required for amplification. Furthermore, no product is observed when only the T7 Sequenase is present in the reaction, demonstrating that a DNA polymerase alone is insufficient to support amplification (Figure 5A, lane 5). Replacement of the T7 Gp2.5 SSB protein with equal amounts of the T4 Gp32 SSB did not support amplification, suggesting that the specific interaction between the T7 replication proteins is essential for cHDA to proceed (Figure 5A, lane 6).
Figure 5.
Analysis of the cHDA reaction components. The cHDA reactions shown here were performed with pREP (6 kb) as a template and S1233 and S1224 as primers. (A) Assessing required cHDA reaction components. Reactions were performed with the proteins indicated in the figure; ‘+’ and ‘−’ denote the presence and absence, respectively, of each indicated protein. Lane 1 is a positive control containing all proteins. In lane 6, the T7 SSB protein was substituted with an equal amount of the T4 SSB protein. Lane M, 2-log DNA ladder. (B) Exponential cHDA amplification requires two primers. Reactions were set up with either two primers (lane 1) or only one primer (land 2 and 3). Lane M, 2-log DNA ladder. (C) Determining the effect of an initial heat denaturation step. The cHDA reactions were performed with pREP as a template with (lanes 1–3) or without (lanes 4 and 5) an initial heat denaturation of the template DNA. The amount of pREP used in the reactions was 15 ng (lanes 1 and 4), 7.5 ng (lanes 2 and 5) or 3.8 ng (lanes 3 and 6).
Amplification of the circular DNA template by cHDA is performed with two specific primers. The requirement of both primers in cHDA was examined by determining the ability of cHDA to proceed in the presence of only one primer. Parallel cHDA reactions containing the pREP plasmid as a template were performed in which one reaction had both primers and, in the two remaining reactions, only one of each primer was present. As shown in Figure 5B, both the specific 2.3 kb fragment and large molecular weight products were only amplified in the presence of two primers (lane 1). When only one primer was used, no corresponding amplification products were observed (Figure 5B, lanes 2 and 3). Therefore, efficient amplification in cHDA is dependent on the presence of the complete T7 replisome, consisting of helicase, DNA polymerase and SSB protein, and two specific primers.
Isothermal amplification methods, such as SDA and multiply primed RCA, frequently require an initial heat denaturation step (2,3). To investigate the effect of a prior denaturation step, two sets of cHDA reactions were simultaneously conducted (Figure 5C): One set was continuously incubated at 25°C for 6 h (one-step amplification), while the second set contained the template DNA that was initially denatured at 95°C for 3 min prior to the addition of other cHDA components and incubation at 25°C for 6 h (two-step amplification). No significant difference was observed in the amplification yield between one- and two-step amplification when enough template DNA was provided (Figure 5C, lanes 1 and 4). However, the initial denaturation step increased the amplification yield when the amount of template DNA was limited (Figure 5C, lanes 2, 3, 5 and 6).
Sensitivity and specificity of cHDA
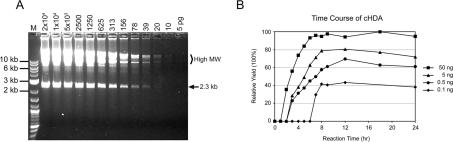
We also investigated the sensitivity of cHDA using varying amounts of template DNA. The pREP DNA was serially diluted and denatured at 95°C for 3 min before introduced to cHDA reactions. After a 6 h incubation at 25°C, a similar amplification yield was obtained from the cHDA reactions containing 20–2.5 ng of the pREP DNA (Figure 6A). The amplification was detected in the cHDA reaction with as little as 20 pg of template DNA, although the final yield was decreased accordingly when an amount <2.5 ng of template DNA was used. In order to calculate the amplification fold of the cHDA reactions, the amount of the 2.3 kb specific product was calculated by using the amount of the DNA marker bands as a reference and divided by the amount of the 2.3 kb region in the template DNA. The amplification fold reached up to 3.6 × 103 when 156 pg of total template DNA was used. Since cHDA produces not only a specific product but also concatemers of the circular template, which carry multiple copies of the specific product, the actual amplification fold would be much higher. When the same template and primer pairs were used in PCR, the amplification fold of the PCR reactions reached up to 4 × 104 when 10 pg of total template DNA was used, and the amplification was detected in the PCR reaction with as little as 1 pg of template DNA (data not shown).
Figure 6.
Sensitivity and yield of cHDA. (A) The sensitivity to the template amount of cHDA was tested. The amount of the pREP plasmid DNA (6 kb) used in each cHDA reaction is indicated on the top. Lane M, 2-log DNA maker (NEB). (B) Time course of cHDA product formation. A series of cHDA reactions containing different amounts of the pREP plasmid template (6 kb; 50, 5, 0.5 or 0.1 ng) was incubated at 25°C for upto 24 h and the incubation was stopped at various time points. The product formation was visualized by agarose gel electrophoresis, and analyzed with a GelDoc system (Bio-Rad). The relative yield (%) of the 2.3 kb specific product was calculated.
To find out if the low yield of cHDA at low template concentrations can be increased by a longer incubation time, time course experiments were carried out. Various amounts of pREP DNA were used in cHDA reactions and the amplification yield was analyzed at various time points. As shown in Figure 6B, the maximum yield of a cHDA reaction is achieved after 6–8 h of incubation regardless of the template amount used.
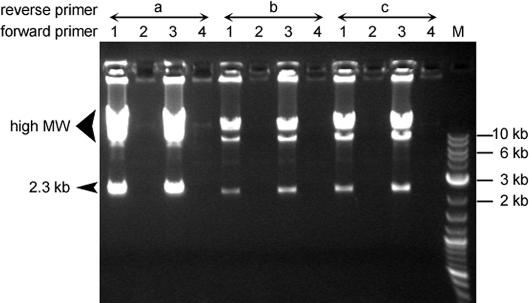
Next, the amplification specificity of cHDA was determined by testing a variety of primers. A series of cHDA reactions were prepared with a pair of primers: forward primers were selected from primers S1201, S1226, S1233 or S1249 (Figure 7, lanes 1, 2, 3 or 4, respectively; Table 1) and a reverse primer was chosen from primers S1224, S1248 or S1212 (Figure 7, lanes a, b or c, respectively; Table 1). Two forward primers, S1226 and S1249, do not have a complementary region in the template and, as expected, the reactions containing either S1226 or S1249 as a forward primer failed to amplify pREP (Figure 7, lanes 2s and 4s). On the other hand, although there was a variation in the amplification yield depending on the primers used, all the cHDA reactions generated amplification products as long as two specific primers were provided to the cHDA reactions (Figure 7, lanes 1s and 3s).
Figure 7.
Primer specificity of cHDA. Various primers were used in the cHDA reactions using pREP (6 kb) as a template. Each cHDA reaction contained one forward primer selected from S1201 (lanes 1), S1226 (lanes 2), S1233 (lanes 3) and S1249 (lanes 4) and one reverse primer chosen from S1224 (lanes a), S1248 (lanes b) and S1212 (lanes c). All of the primers carry sequences complementary to the pREP seqnence except two forward primers S1226 and S1249, and amplify the same region. The size of specific products is similar (2.3 kb). Lane M, 2-log DNA marker (NEB).
Colony amplification of plasmids by cHDA
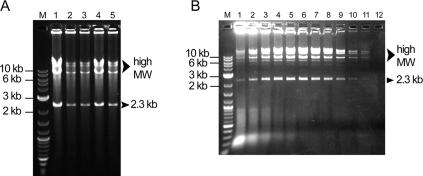
The ability of the cHDA system to efficiently amplify plasmids directly from bacterial cells without plasmid purification was assessed. The pREP plasmid DNA was used to transform the E.coli strain ER2502 (NEB), and five individual transformant colonies were randomly selected for testing. Bacterial cells from each colony were suspended in a cHDA reaction mix containing all the components except T7 proteins. The cells were lysed by heating at 95°C for 3 min. After being mixed with the T7 proteins, the reaction was incubated at 25°C for 6 h. As shown in Figure 8A, although the amplification yields of five colony cHDA reactions were different, all of the reactions were able to amplify the 2.3 kb specific product and plasmid concatemers (lanes 1–5) previously observed for cHDA reactions performed with the purified plasmid. Amplification products from colony cHDA reactions were sequenced and confirmed that the pREP plasmid was specifically amplified (data not shown).
Figure 8.
Amplification of crude cell lysates by cHDA. (A) Cells harboring pREP (6 kb) were separately collected from five individual colonies (lanes 1–5) randomly selected from a transformation plate and used in the cHDA reactions after incubation at 95°C for 3 min. Lane M, 2-log DNA marker (NEB). (B) Determining detection sensitivity of cHDA. Parallel reactions were performed using resuspended cells, harboring pREP (6 kb), which were serially diluted and plated to determine the cell density. Lanes are as follows: lane M, 2-log DNA ladder; lane 1, 6 × 106; lane 2, 3 × 106; lane 3, 1.5 × 106; lane 4, 7.7 × 105; lane 5, 3.9 × 105; lane 6, 1.9 × 105; lane 7, 9.6 × 104; lane 8, 4.8 × 104; lane 9, 2.4 × 104; lane 10, 1.2 × 104; lane 11, 6000; and lane 12, 3000 cells.
The sensitivity of colony cHDA was examined by introducing serially diluted cell suspensions to the reactions. Colony cells were resuspended in dH2O and a serial dilution was made. An equal amount of each dilution was either used in a cHDA reaction or plated to determine the number of cells used in the reaction. Products were detected from as few as 6000 cells harboring pREP (Figure 8B): without amplification, no DNA with a size similar to the amplification products was detected (data not shown). Maximum amplification was observed at 1.9 × 105 cells (Figure 8B, lane 6), which is equivalent to a half of one colony. Above 7.7 × 105 cells, amplification efficiency diminished suggesting that cell lysate contaminants became inhibitory. These results demonstrate that cHDA can be used to accurately screen bacterial colonies for plasmids and that plasmid DNA is selectively amplified in the presence of genomic DNA.
Template preference of cHDA
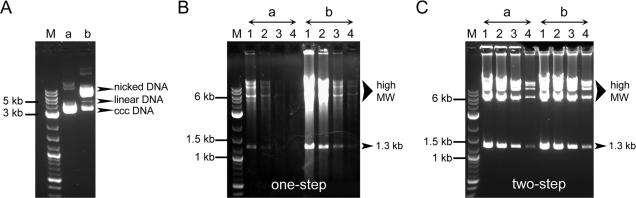
The cHDA system is capable of amplifying a very long DNA fragment using a circular DNA molecule as a template. The plasmid DNA used in cHDA is mainly in the form of covalently closed circular DNA (cccDNA) with a small amount of DNA in a nicked circular form. To determine which form of circular DNA serves as a better template for cHDA, we tested either cccDNA or nicked cDNA in a cHDA reaction. The nicked form of circular DNA template was prepared by converting most of cccDNA (Figure 9A, lanes a) to nicked cDNA (Figure 9A, lanes b) by incubating the pCR-4E plasmid DNA with a nicking restriction endonuclease, BbvCIB. When the cccDNA or nicked cDNA templates were used in one-step amplification of cHDA, the cHDA reactions with the nicked cDNA template showed a robust amplification compared to those with the cccDNA template (Figure 9B). This result suggests that the efficient amplification of cHDA relies on having nicked cDNA as a template. However, this limitation can be overcome by denaturing the DNA template before amplification. As shown in Figure 9C, by denaturing the circular DNA template, the cccDNA or nicked cDNA templates demonstrated similar amplification efficiency in cHDA. We also tested if the cHDA system can use linear duplex DNA as a template, i.e. a PCR-type amplification. The linear duplex DNA was generated either by digesting circular plasmid DNA with restriction endonucleases (primers anneal in the middle of the template) or by PCR with the same primer pair used in the cHDA reaction (primers anneal to the end of the template), and used in cHDA as a template. However, cHDA could not amplify from the linear templates (data not shown).
Figure 9.
Template preference of cHDA. Two different forms of circular DNA, cccDNA or nicked cDNA, were compared as a template in the cHDA reactions. Lane M, 2-log DNA marker (NEB). The pCR-4E plasmid DNA (4.5 kb) in the form of cccDNA (A: lane a) was converted to a nicked cDNA form by BbvCIB digestion (A: lane b). The one-step (B) or two-step (C) cHDA reactions were performed using PCR-4E as a template and S1233 and S1224 as primers: lanes a, cccDNA: lanes b, nicked DNA. The amount of pCR-4E used was 10 ng (lanes 1), 2.5 ng (lanes 2), 0.6 ng (lanes 3) or 0.16 ng (lanes 4).
DISCUSSION
HDA exploits the cell's replication machinery to mimic the process of duplicating genomic DNA in vivo. During DNA replication, a helicase unwinds DNA to provide single-stranded templates for polymerase-dependent DNA synthesis (22). Similarly, HDA reactions rely on the coordinated activities of helicase, SSB proteins and DNA polymerase to amplify DNA. Helicase melts the DNA template and SSB proteins coordinate replication, allowing DNA polymerase to extend annealed primers (4). A new HDA platform, cHDA, has been developed using the replicative machinery of bacteriophage T7 that can replicate its 40 kb genome in one initiation event (5,6). The cHDA system uses a modified T7 replisome, composed of T7 Sequenase, 4B helicase and Gp2.5 SSB protein, to perform helicase-dependent and strand-displacement amplification. The T7 helicase, like other replicative helicases, displays a rapid translocation rate and high processivity, traveling 75 kb at a rate of 130 nt/s on ssDNA before dissociating (16). Using circular DNA as a template, the cHDA sytem is able to amplify both a target region defined by specific primers and the entire circular template harboring the target DNA.
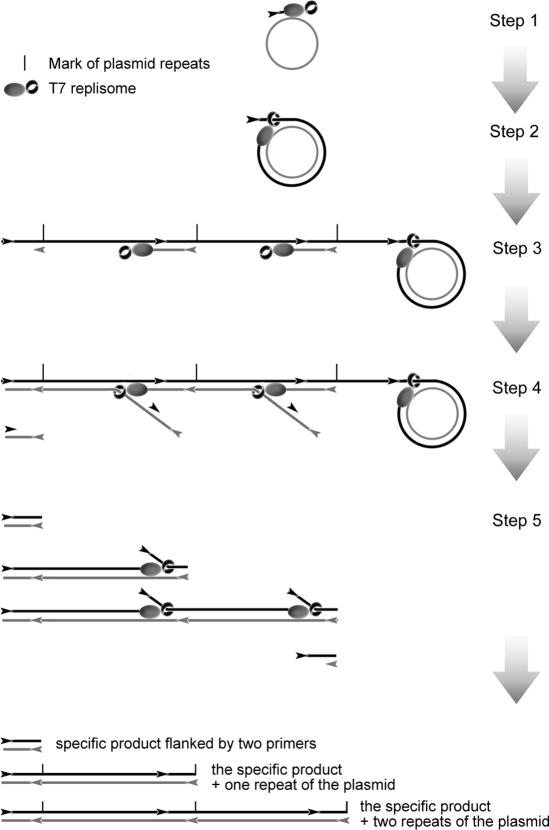
Based on the results from this study, the amplification mechanism of cHDA was postulated to be a helicase-dependent RCA, as illustrated in Figure 10. Amplification is initiated by the annealing of a forward primer to a single-stranded circle or to one of the two circular strands of the plasmid. For simplicity, the primer is shown as annealed to a single-stranded circle in Step 1 of Figure 10. The primer is extended by the T7 replisome, which includes the T7 Sequenase, T7 gp4B helicase and T7 gp2.5 SSB protein; The SSB protein is omitted from the figure to simplify the diagram. When replication reaches the 5′ end of the newly synthesized strand, helicase binds to the 5′ end and the replisome begins strand displacement synthesis around the circular template (Step 2). The displaced tail grows and provides multiple sites for reverse primers to anneal and to be extended by T7 replisomes (Step 3). Primer extension continues until the replisome reaches the duplex ahead, and begins strand displacement synthesis, releasing ssDNA products, which carry one or more sites for forward primers to bind (Step 4). The forward primers are extended by T7 replisomes, resulting in the synthesis of dsDNA products and release of ssDNA for the next round of strand displacement synthesis (Step 5). Multiple rounds of strand displacement synthesis by the T7 replisome produce a specific product defined by two primers and concatemers of the circular DNA template. The larger products increase in size by the unit size of the circular template present in the concatamers. Based on published studies on T7 replication [reviewed in (10)], we can speculate the role of each T7 protein in the cHDA system: during the amplification process, helicases unwind duplex DNA ahead of the DNA polymerase; SSB protein stabilizes the transient ssDNAs unwound by the helicase by preventing the reassociation of duble-stranded DNA, thus allowing primers to anneal to the template; polymerase extends the primers. When cHDA is used with a double-stranded circular DNA as a template, the same reaction is carried out on the complementary strand of the circle, beginning with a reverse primer annealed to the template. Therefore, ssDNA products generated from the forward primers can anneal to the ssDNA produced from the reverse primers, which may be an additional source for generating dsDNA products.
Figure 10.
Schematic diagram of a cHDA reaction. A single-stranded circular DNA template is shown as a grey circle and primers are indicated as arrowheads. T7 replisome consists of T7 DNA polymerase-thioredoxin complex (grey oval) and T7 Gp 4B helicase (black ring): T7 Gp2.5 SSB has been omitted to simplify the diagram. Step 1, a forward primer anneals to the complementary region within the single-stranded circular template and the T7 replisome extends the primer; Step 2, the 5′ end of the newly synthesized strand is displaced by the T7 replisome at the extending 3′ end; Step 3, rolling circle synthesis continues and the displaced strand provides multiple sites for reverse primers to anneal and to be extended by T7 replisomes; Step 4, ssDNA molecules are released by T7 replisomes performing strand displacement synthesis from the reverse primers annealed upstream, and they provide complementary sites for forward primers to bind; Step 5, the forward primers are extended by T7 replisomes, resulting in the synthesis of dsDNA products and release of ssDNA for the next round of strand displacement synthesis. Multiple rounds of strand displacement synthesis by the T7 replisome produce a specific product defined by two primers and concatemers of the circular DNA template.
Amplification by cHDA is dependent on the presence and specific interaction of the SSB protein, helicase and T7 Sequenase. Without the T7 helicase and/or SSB protein, no product is observed with only the T7 Sequenase. In the presence of all three proteins, plasmid inserts of up to 10 kb can be amplified from bacterial colonies, bypassing a DNA purification step. Substitution of T7 gp2.5 with the T4 gp32 SSB protein (Figure 5A) or E.coli SSB protein (data not shown) does not support cHDA amplification. The T7 SSB protein displays a high annealing activity that promotes base pairing between complementary sequences (23). In a cHDA reaction, primers are highly abundant and predicted to be coated with the SSB protein. It is conceivable that the presence of the SSB protein mediates homologous base pairing to stimulate primer annealing and opening at the end of dsDNA to provide an entry point for the T7 helicase, which prefers forked duplex DNA with single-stranded tails (12,24). Binding of the SSB protein to the unwound ssDNA produced by the helicase is expected to stabilize each strand and to inhibit reannealing. In addition, the T7 Gp2.5 SSB interacts with T7 DNA polymerase and stimulates DNA synthesis by T7 DNA polymerase (19,20,25). It also stimulates the synthesis of RNA primers by T7 helicase and was suggested to interact with T7 helicase (24). Furthermore, T7 helicase and T7 DNA polymerase form a complex and both proteins are required for DNA synthesis through a duplex region (26–29).
The strand displacement activity of T7 Sequenase is central to the cHDA platform, supporting displacement of the newly synthesized strand. The wild-type T7 polymerase lacks strand displacement activity and is unable to support cHDA (data not shown). Due to the absence of the 3′–5′ exonuclease activity, the T7 Sequenase used in cHDA has a lower fidelity (error rate of 3.6 × 10−5) than the wild-type T7 polymerase, which has a error rate of 1.5 × 10−6 (30,31). One approach to further increase the fidelity is to combine T7 Sequenase with a DNA polymerase possessing proofreading activity in cHDA reactions. Another approach is to explore the use of the T7 SSB protein mutant allele, F232L, which has been shown to promote strand displacement activity in the native T7 polymerase (32). The F232L SSB protein has also been demonstrated to possess a higher binding affinity for ssDNA and to stimulate T7 DNA polymerase activity (32). Developing a cHDA system that utilizes the F232L SSB protein may allow the use of the wild-type T7 DNA polymerase and will improve the potential error rate associated with the T7 Sequenase enzyme.
Currently, an isothermal plasmid amplification system utilizing the Φ29 polymerase is available for research use (GE Healthcare). Development of the T7 bacteriophage based cHDA system overcomes many of the drawbacks associated with the Φ29 system, allowing a more efficient isothermal plasmid amplification. Amplification with the Φ29 system requires heat denaturation of the template DNA at 95°C, followed by incubation at 30°C for the reaction to proceed; therefore, the system is not a true isothermal reaction. The use of helicase in cHDA reactions eliminates the need for temperature cycling to facilitate access to the template sequence by enzymatically melting duplex DNA. Furthermore, primers in the Φ29 system are random hexamers and, in contrast, only sequence specific primers are utilized in cHDA. Multiply primed random hexamers mediate efficient amplification, but also render the system sensitive to the presence of contaminating DNAs. Additionally, the product from the Φ29 platform is a hyperbranched and viscous. In the event that the Φ29 system is being used for plasmid analysis, an additional screening step is necessary to determine the identity of the amplified plasmids.
Researchers typically rely on E.coli cells to amplify plasmid DNA, by growing cultures of transformed cells harboring the plasmid and purifying the DNA. This technique can be time consuming and labor intensive when analyzing numerous isolates. A faster, isothermal method that selectively amplifies the target of interest for analyzing plasmids at room temperature has the potential to reduce the time necessary for analyzing DNA constructs and facilitate high throughput genetic screens. The unmatched ability of the cHDA system to simultaneously amplify plasmids and screen for specific inserts reduces plasmid analysis procedures to one time saving reaction. The true isothermal nature of cHDA will also allow performing specific DNA amplification where a thermocycler is not readily available. In addition, since cHDA specifically amplifies circular DNA, this technology will be useful when circular DNA needs to be amplified in the presence of contaminating linear DNA. For example, the cHDA technology can be further developed to preferentially amplify mitochondrial DNA from human cells for further genetic analysis, or to detect the presence of circular DNA virus in a clinical specimen or a specific plasmid in the pathogenic bacteria.
Acknowledgments
We are grateful to Jeremy Foster, Lauren Higgins, Myriam Vincent, Jamie Wytiaz and Michael Dalton for their technical support during the project. We thank Richard Roberts and Bertrand Lemieux for the discussion and critical reading of the manuscript. We also thank New England Biolabs for providing the enzymes and reagents as well as oligonucleotides used in this study. This work was supported by National Institutes of Health SBIR Grant GM073402. Funding to pay the Open Access publication charges for this article was provided by NIH.
Conflict of interest statement. None declared.
REFERENCES
- 1.Sambrook J., Russell D.W. In vitro Amplification of DNA by the Polymerase Chain Reaction. In: Sambrook J., Russell D.W., editors. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2001. pp. 8.1–8.126. [Google Scholar]
- 2.Walker G.T., Little M.C., Nadeau J.G., Shank D.D. Isothermal in vitro amplification of DNA by a restriction enzyme/DNA polymerase system. Proc. Natl Acad. Sci. USA. 1992;89:392–396. doi: 10.1073/pnas.89.1.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean F.B., Nelson J.R., Giesler T.L., Lasken R.S. Rapid amplification of plasmid and phage DNA using Phi 29 DNA polymerase and multiply-primed rolling circle amplification. Genome Res. 2001;11:1095–1099. doi: 10.1101/gr.180501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincent M., Xu Y., Kong H. Helicase-dependent isothermal DNA amplification. EMBO Rep. 2004;5:795–800. doi: 10.1038/sj.embor.7400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debyser Z., Tabor S., Richardson C.C. Coordination of leading and lagging strand DNA synthesis at the replication fork of bacteriophage T7. Cell. 1994;77:157–166. doi: 10.1016/0092-8674(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 6.Lee J., Chastain P.D., II, Kusakabe T., Griffith J.D., Richardson C.C. Coordinated leading and lagging strand DNA synthesis on a minicircular template. Mol. Cell. 1998;1:1001–1010. doi: 10.1016/s1097-2765(00)80100-8. [DOI] [PubMed] [Google Scholar]
- 7.Tabor S., Huber H.E., Richardson C.C. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J. Biol. Chem. 1987;262:16212–16223. [PubMed] [Google Scholar]
- 8.Tabor S., Richardson C.C. Selective inactivation of the exonuclease activity of bacteriophage T7 DNA polymerase by in vitro mutagenesis. J. Biol. Chem. 1989;264:6447–6458. [PubMed] [Google Scholar]
- 9.Dunn J.J., Studier F.W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J. Mol. Biol. 1983;166:477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- 10.Richardson C.C. Bacteriophage T7: minimal requirements for the replication of a duplex DNA molecule. Cell. 1983;33:315–317. doi: 10.1016/0092-8674(83)90411-7. [DOI] [PubMed] [Google Scholar]
- 11.Tabor S., Richardson C.C. Template recognition sequence for RNA primer synthesis by gene 4 protein of bacteriophage T7. Proc. Natl Acad. Sci. USA. 1981;78:205–209. doi: 10.1073/pnas.78.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matson S.W., Tabor S., Richardson C.C. The gene 4 protein of bacteriophage T7. Characterization of helicase activity. J. Biol. Chem. 1983;258:14017–14024. [PubMed] [Google Scholar]
- 13.Mendelman L.V., Beauchamp B.B., Richardson C.C. Requirement for a zinc motif for template recognition by the bacteriophage T7 primase. EMBO J. 1994;13:3909–3916. doi: 10.1002/j.1460-2075.1994.tb06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein J.A., Richardson C.C. Purification of the 56-kDa component of the bacteriophage T7 primase/helicase and characterization of its nucleoside 5′-triphosphatase activity. J. Biol. Chem. 1988;263:14891–14899. [PubMed] [Google Scholar]
- 15.Bernstein J.A., Richardson C.C. A 7-kDa region of the bacteriophage T7 gene 4 protein is required for primase but not for helicase activity. Proc. Natl Acad. Sci. USA. 1988;85:396–400. doi: 10.1073/pnas.85.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D.E., Narayan M., Patel S.S. T7 DNA helicase: a molecular motor that processively and unidirectionally translocates along single-stranded DNA. J. Mol. Biol. 2002;321:807–819. doi: 10.1016/s0022-2836(02)00733-7. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y.T., Tabor S., Bortner C., Griffith J.D., Richardson C.C. Purification and characterization of the bacteriophage T7 gene 2.5 protein. A single-stranded DNA-binding protein. J. Biol. Chem. 1992;267:15022–15031. [PubMed] [Google Scholar]
- 18.Hollis T., Stattel J.M., Walther D.S., Richardson C.C., Ellenberger T. Structure of the gene 2.5 protein, a single-stranded DNA binding protein encoded by bacteriophage T7. Proc. Natl Acad. Sci. USA. 2001;98:9557–9562. doi: 10.1073/pnas.171317698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherzinger E., Litfin F., Jost E. Stimulation of T7 DNA polymerase by a new phage-coded protein. Mol. Gen. Genet. 1973;123:247–262. doi: 10.1007/BF00271243. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y.T., Tabor S., Churchich J.E., Richardson C.C. Interactions of gene 2.5 protein and DNA polymerase of bacteriophage T7. J. Biol. Chem. 1992;267:15032–15040. [PubMed] [Google Scholar]
- 21.Nakai H., Richardson C.C. Leading and lagging strand synthesis at the replication fork of bacteriophage T7. Distinct properties of T7 gene 4 protein as a helicase and primase. J. Biol. Chem. 1988;263:9818–9830. [PubMed] [Google Scholar]
- 22.Kornberg A., Baker T.A. DNA helicases. In: Kornberg A., Baker T., editors. DNA Replication. 2nd edn. New York: W.H. Freeman and Company; 1992. pp. 355–378. [Google Scholar]
- 23.Kong D., Richardson C.C. Single-stranded DNA binding protein and DNA helicase of bacteriophage T7 mediate homologous DNA strand exchange. EMBO J. 1996;15:2010–2019. [PMC free article] [PubMed] [Google Scholar]
- 24.Nakai H., Richardson C.C. The effect of the T7 and Escherichia coli DNA-binding proteins at the replication fork of bacteriophage T7. J. Biol. Chem. 1988;263:9831–9839. [PubMed] [Google Scholar]
- 25.Kim Y.T., Richardson C.C. Acidic carboxyl-terminal domain of gene 2.5 protein of bacteriophage T7 is essential for protein–protein interactions. J. Biol. Chem. 1994;269:5270–5278. [PubMed] [Google Scholar]
- 26.Engler M.J., Lechner R.L., Richardson C.C. Two forms of the DNA polymerase of bacteriophage T7. J. Biol. Chem. 1983;258:11165–11173. [PubMed] [Google Scholar]
- 27.Lechner R.L., Richardson C.C. A preformed, topologically stable replication fork. Characterization of leading strand DNA synthesis catalyzed by T7 DNA polymerase and T7 gene 4 protein. J. Biol. Chem. 1983;258:11185–11196. [PubMed] [Google Scholar]
- 28.Nakai H., Richardson C.C. Interactions of the DNA polymerase and gene 4 protein of bacteriophage T7. Protein–protein and protein–DNA interactions involved in RNA-primed DNA synthesis. J. Biol. Chem. 1986;261:15208–15216. [PubMed] [Google Scholar]
- 29.Notarnicola S.M., Mulcahy H.L., Lee J., Richardson C.C. The acidic carboxyl terminus of the bacteriophage T7 gene 4 helicase/primase interacts with T7 DNA polymerase. J. Biol. Chem. 1997;272:18425–18433. doi: 10.1074/jbc.272.29.18425. [DOI] [PubMed] [Google Scholar]
- 30.Keohavong P., Thilly W.G. Fidelity of DNA polymerases in DNA amplification. Proc. Natl Acad. Sci. USA. 1989;86:9253–9257. doi: 10.1073/pnas.86.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mattila P., Korpela J., Tenkanen T., Pitkanen K. Fidelity of DNA synthesis by the Thermococcus litoralis DNA polymerase—an extremely heat stable enzyme with proofreading activity. Nucleic Acids Res. 1991;19:4967–4973. doi: 10.1093/nar/19.18.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Z.G., Rezende L.F., Willcox S., Griffith J.D., Richardson C.C. The carboxyl-terminal domain of bacteriophage T7 single-stranded DNA-binding protein modulates DNA binding and interaction with T7 DNA polymerase. J. Biol. Chem. 2003;278:29538–29545. doi: 10.1074/jbc.M304318200. [DOI] [PubMed] [Google Scholar]