Abstract
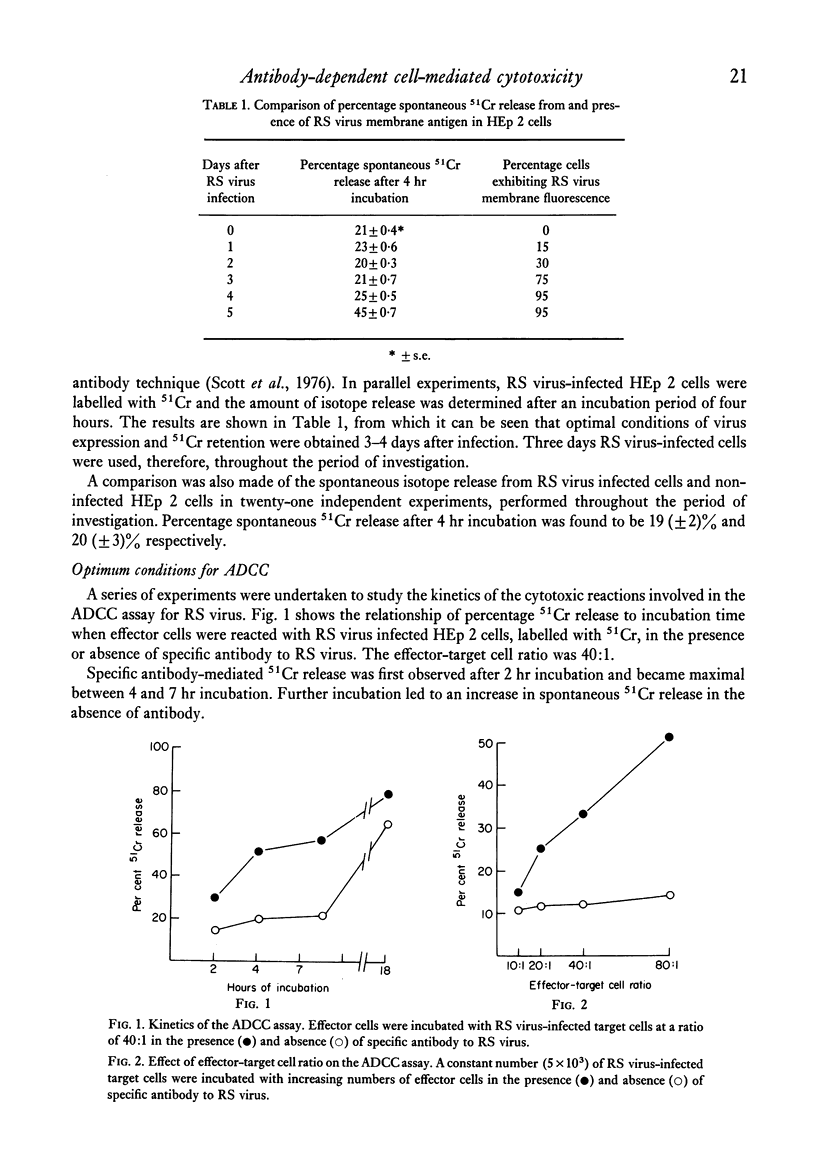
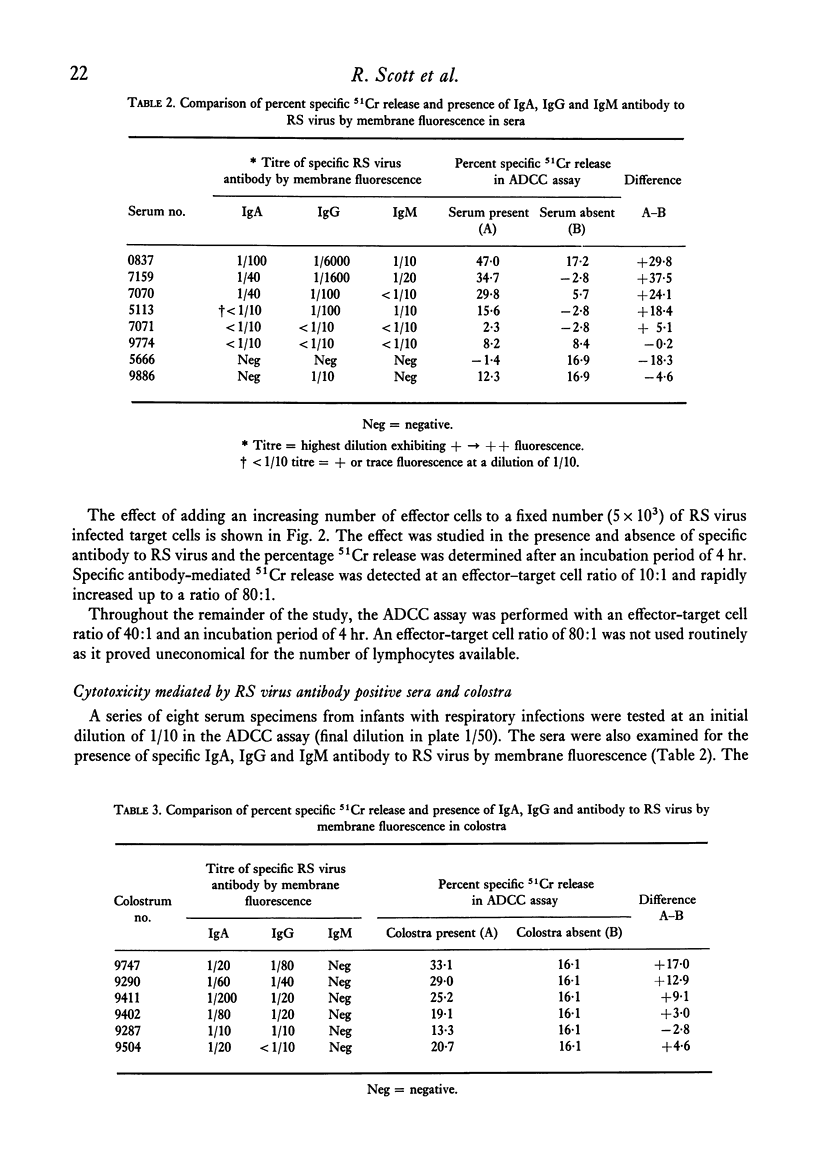
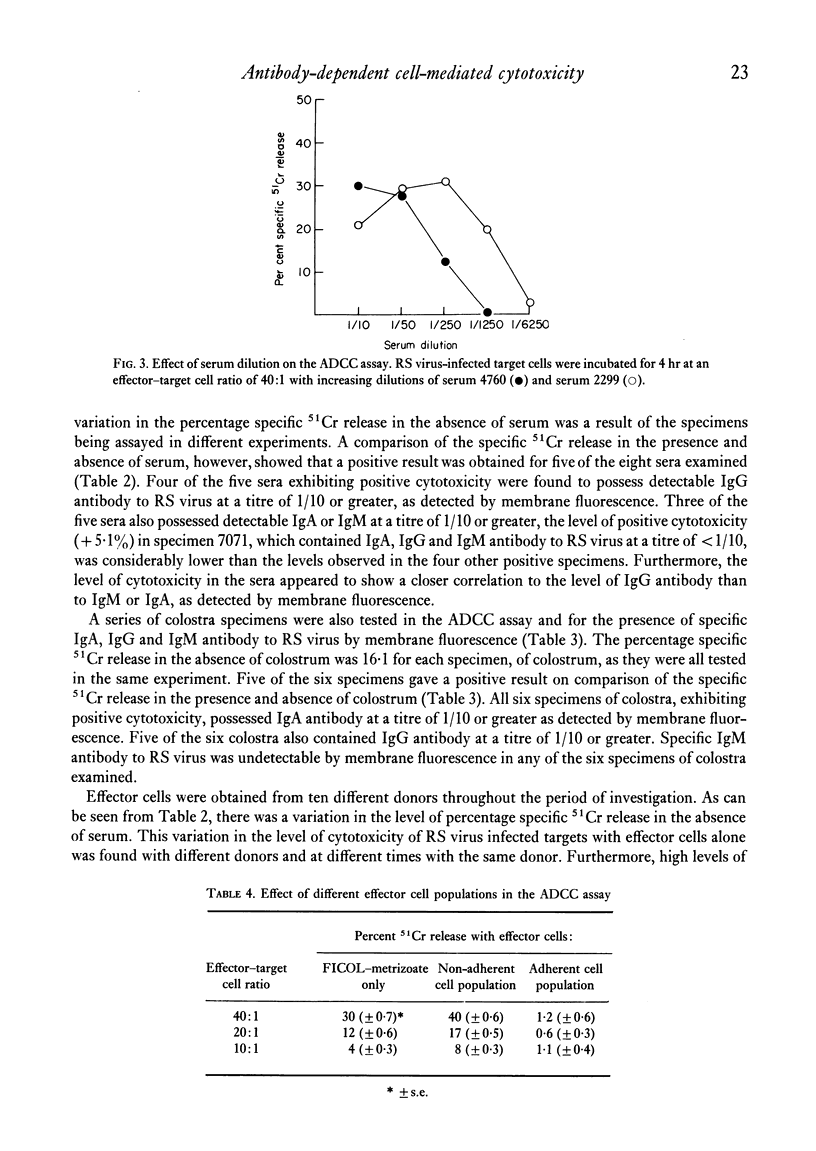
A chromium release assay was established to study human antibody-dependent cell-mediated cytotoxicity (ADCC) of HEp 2 cells infected with respiratory syncytial (RS) virus. Human peripheral blood lymphocytes in the presence of specific antibody to RS virus caused in vitro lysis of RS virus infected target cells. ADCC was detected in sera of infants with RS virus infections and in specimens of colostrum. The ability of serum or colostrum to mediate the cytotoxic reaction appeared to be related to the level of specific IgG, or IgA antibody to RS virus, as detected by membrane fluorescence. Separation of effector cells by their glass adherence properties showed that the ability to produce cytotoxicity resided in non-adherent effector cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson E., Birchall J. P., Owen J. J. A simple method of lymphocyte purification from human peripheral blood. J Immunol Methods. 1976;11(3-4):297–301. doi: 10.1016/0022-1759(76)90123-x. [DOI] [PubMed] [Google Scholar]
- Allison A. C. Immunity and immunopathology in virus infections. Ann Inst Pasteur (Paris) 1972 Oct;123(4):585–608. [PubMed] [Google Scholar]
- CHANOCK R. M., KIM H. W., VARGOSKO A. J., DELEVA A., JOHNSON K. M., CUMMING C., PARROTT R. H. Respiratory syncytial virus. I. Virus recovery and other observations during 1960 outbreak of bronchiolitis, pneumonia, and minor respiratory diseases in children. JAMA. 1961 May 27;176:647–653. [PubMed] [Google Scholar]
- Downham M. A., Scott R., Sims D. G., Webb J. K., Gardner P. S. Breast-feeding protects against respiratory syncytial virus infections. Br Med J. 1976 Jul 31;2(6030):274–276. doi: 10.1136/bmj.2.6030.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P. S. Virus infections and respiratory disease of childhood. Arch Dis Child. 1968 Dec;43(232):629–645. doi: 10.1136/adc.43.232.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härfast B., Andersson T., Perlmann P. Human lymphocyte cytotoxicity against mumps virus-infected target cells. Requirement for non-T cells. J Immunol. 1975 Jun;114(6):1820–1823. [PubMed] [Google Scholar]
- Jondal M. Antibody-dependent cellular cytotoxicity (ADCC) against Epstein-Barr virus-determined membrane antigens. I. Reactivity in sera from normal persons and from patients with acute infectious mononucleosis. Clin Exp Immunol. 1976 Jul;25(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Lamon E. W., Skurzak H. M., Andersson B., Whitten H. D., Klein E. Antibody-dependent lymphocyte cytotoxicity in the murine sarcoma virus system: activity of IgM and IgG with specificity for MLV determined antigen(s). J Immunol. 1975 Apr;114(4):1171–1176. [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Bonnard G. D., Sordat B., Zawodnik S. A. Antibody-dependent cell-mediated cytotoxicity: heterogeneity of effector cells in human peripheral blood. Scand J Immunol. 1975 Sep;4(5-6):487–497. doi: 10.1111/j.1365-3083.1975.tb02654.x. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C. Antibody in the induction and inhibition of lymphocyte cytotoxicity. Transplant Rev. 1972;13:67–90. doi: 10.1111/j.1600-065x.1972.tb00060.x. [DOI] [PubMed] [Google Scholar]
- MacLennan I. C., Loewi G., Howard A. A human serum immunoglobulin with specificity for certain homologous target cells, which induces target cell damage by normal human lymphocytes. Immunology. 1969 Dec;17(6):897–910. [PMC free article] [PubMed] [Google Scholar]
- Ortiz de Landazuri M., Kedar E., Fahey J. L. Antibody-dependent cellular cytotoxicity to a syngeneic Gross virus-induced lymphoma. J Natl Cancer Inst. 1974 Jan;52(1):147–152. doi: 10.1093/jnci/52.1.147. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Orr T. W. Antibody-dependent lymphocyte cytotoxicity against cells expressing Epstein-Barr virus antigens. J Natl Cancer Inst. 1976 Mar;56(3):485–488. doi: 10.1093/jnci/56.3.485. [DOI] [PubMed] [Google Scholar]
- Perlmann P., Holm G. Cytotoxic effects of lymphoid cells in vitro. Adv Immunol. 1969;11:117–193. doi: 10.1016/s0065-2776(08)60479-4. [DOI] [PubMed] [Google Scholar]
- Pollack S. B., Nelson K., Grausz J. D. Separation of effector cells mediating antibody-dependent cellular cytotoxicity (ADC) to erythrocyte targets from those mediating ADC to tumor targets. J Immunol. 1976 Apr;116(4):944–946. [PubMed] [Google Scholar]
- Rager-Zisman B., Bloom B. R. Immunological destruction of herpes simplex virus I infected cells. Nature. 1974 Oct 11;251(5475):542–543. doi: 10.1038/251542a0. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A. Lysis of herpesvirus-infected cells by immune spleen cells. Infect Immun. 1975 Apr;11(4):767–769. doi: 10.1128/iai.11.4.767-769.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A. Antibody-dependent cell-mediated cytotoxicity in cows: comparison of effector cell activity against heterologous erthrocyte and herpesvirus-infected bovine target cells. Infect Immun. 1976 May;13(5):1433–1441. doi: 10.1128/iai.13.5.1433-1441.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R., De Landazuri M. O., Gardner P. S., Owen J. J. Detection of antibody to respiratory syncytial virus by membrane fluorescence. Clin Exp Immunol. 1976 Oct;26(1):78–85. [PMC free article] [PubMed] [Google Scholar]
- Shore S. L., Black C. M., Melewicz F. M., Wood P. A., Nahmias A. J. Antibody-dependent cell-mediated cytotoxicity to target cells infected with type 1 and type 2 herpes simplex virus. J Immunol. 1976 Jan;116(1):194–201. [PubMed] [Google Scholar]
- Shore S. L., Nahmias A. J., Starr S. E., Wood P. A., McFarlin D. E. Detection of cell-dependent cytotoxic antibody to cells infected with herpes simplex virus. Nature. 1974 Sep 27;251(5473):350–352. doi: 10.1038/251350a0. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Adam E., Melnick J. L., Rawls W. E. Use of the 51 Cr release test to demonstrate patterns of antibody response in humans to herpesvirus types 1 and 2. J Immunol. 1972 Sep;109(3):554–564. [PubMed] [Google Scholar]
- Steele R. W., Hensen S. A., Vincent M. M., Fuccillo D. A., Bellanti J. A. A 51 Cr microassay technique for cell-mediated immunity to viruses. J Immunol. 1973 Jun;110(6):1502–1510. [PubMed] [Google Scholar]


