Abstract
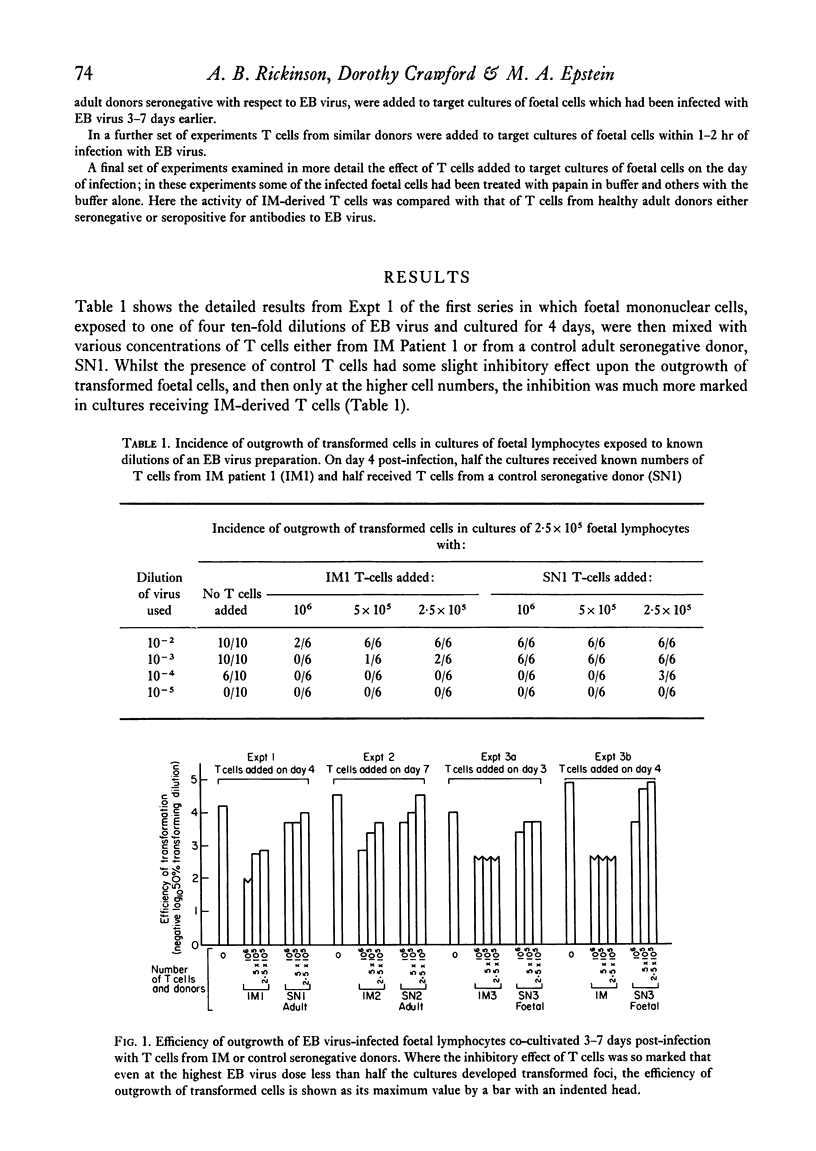
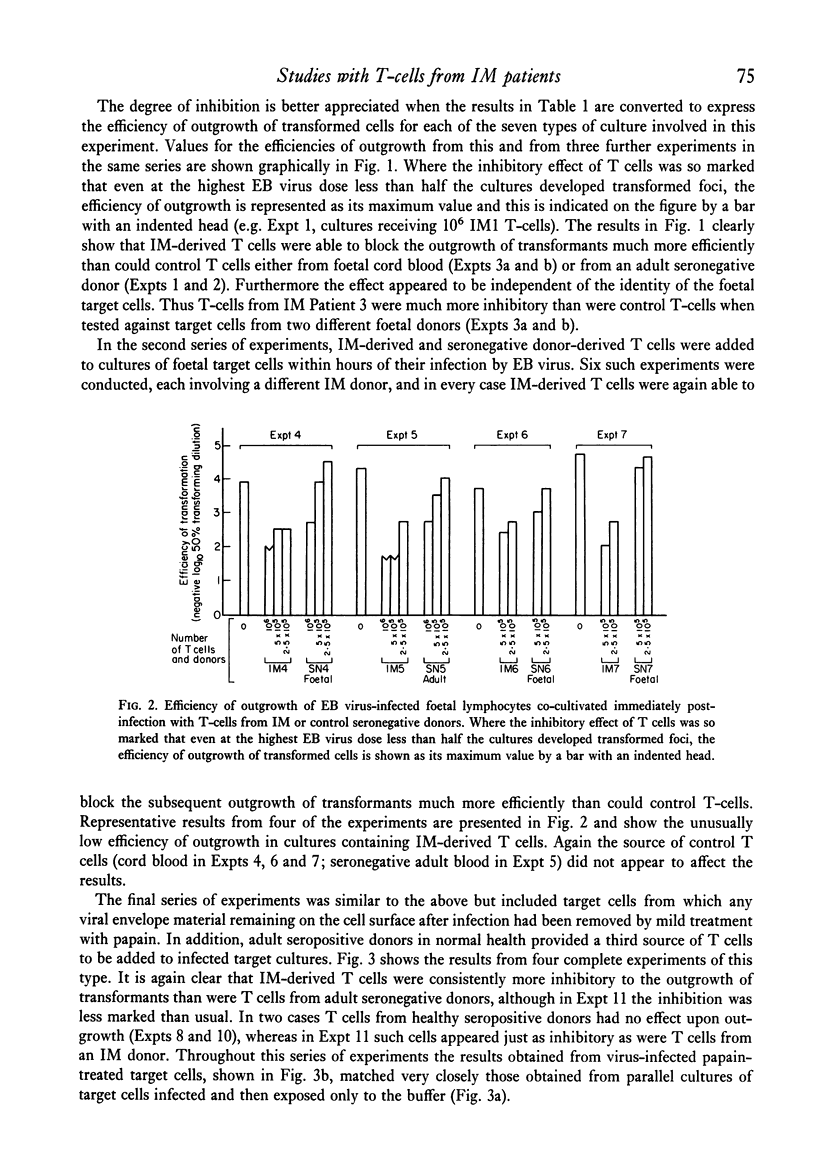
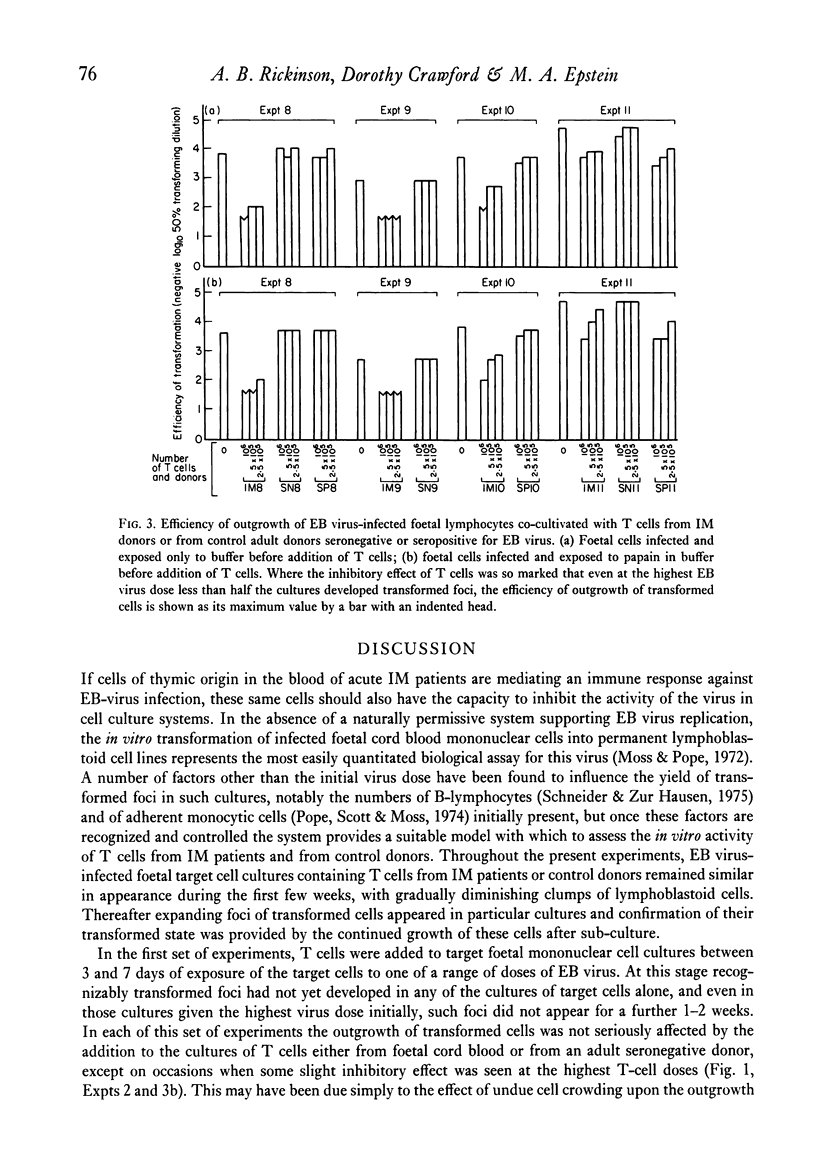
Known numbers of thymus-dependent (T) lymphocytes, obtained by positive selection from the blood of acute infectious mononucleosis (IM) patients and from control donors, were added to target cultures of foetal mononuclear cells within 0-7 days of exposure of the target cells to one of a range of doses of Epstein-Barr (EB) virus. The subsequent outgrowth of virus-transformed foetal cells was markedly inhibited by the presence in the cultures of IM-derived T cells, whilst similar numbers of T cells prepared either from cord blood or from adult donors seronegative for EB virus had little or no inhibitory effect. Target foetal cells treated with papain to remove any viral envelope material remaining on the cell surface after infection, were just as sensitive as untreated cells to the addition of IM-derived T cells. It is concluded that the inhibition cannot be mediated through recognition either of viral envelope structures on the surface of infected cells or of the antigenically related virus-determined membrane antigen, MA, but must depend upon recognition of the lymphocyte-detected membrane antigen, LYDMA. The regularity with which IM-derived T cells block the outgrowth of virus-transformed foetal cells suggests that LYDMA consistently appears on the surface of infected foetal cells before the establishment of transformed foci, but is unlikely to be directly associated with the cells' existing histocompatibility antigens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. Concentration of Epstein-Barr virus from cell culture fluids with polyethylene glycol. J Gen Virol. 1973 Sep;20(3):391–394. doi: 10.1099/0022-1317-20-3-391. [DOI] [PubMed] [Google Scholar]
- Brown G., Greaves M. F. Cell surface markers for human T and B lymphocytes. Eur J Immunol. 1974 Apr;4(4):302–310. doi: 10.1002/eji.1830040414. [DOI] [PubMed] [Google Scholar]
- Dalens M., Zech L., Klein G. Origin of lymphoid lines established from mixed cultures of cord-blood lymphocytes and explants from infectious mononucleosis, Burkitt lymphoma and healthy donors. Int J Cancer. 1975 Dec 15;16(6):1008–1014. doi: 10.1002/ijc.2910160614. [DOI] [PubMed] [Google Scholar]
- Denman A. M., Pelton B. K. Control mechanisms in infectious mononucleosis. Clin Exp Immunol. 1974 Sep;18(1):13–25. [PMC free article] [PubMed] [Google Scholar]
- Dölken G., Klein G. Expression of Epstein-Barr-virus-associated membrane antigen in Raji cells superinfected with two different virus strains. Virology. 1976 Mar;70(1):210–213. doi: 10.1016/0042-6822(76)90255-5. [DOI] [PubMed] [Google Scholar]
- Enberg R. N., Eberle B. J., Williams R. C., Jr T- and B-cells in peripheral blood during infectious mononucleosis. J Infect Dis. 1974 Aug;130(2):104–111. doi: 10.1093/infdis/130.2.104. [DOI] [PubMed] [Google Scholar]
- Ernberg I., Klein G., Kourilsky F. M., Silvestre D. Differentiation between early and late membrane antigen on human lymphoblastoid cell lines infected with Epstein-Barr virus. I. Immunofluorescence. J Natl Cancer Inst. 1974 Jul;53(1):61–65. doi: 10.1093/jnci/53.1.61. [DOI] [PubMed] [Google Scholar]
- Fiala M., Payne J. E., Berne T. V., Moore T. C., Henle W., Montgomerie J. Z., Chatterjee S. N., Guze L. B. Epidemiology of cytomegalovirus infection after transplantation and immunosuppression. J Infect Dis. 1975 Oct;132(4):421–433. doi: 10.1093/infdis/132.4.421. [DOI] [PubMed] [Google Scholar]
- Gerber P., Lucas S., Nonoyama M., Perlin E., Goldstein L. I. Oral excretion of Epstein-Barr virus by healthy subjects and patients with infectious mononucleosis. Lancet. 1972 Nov 11;2(7785):988–989. doi: 10.1016/s0140-6736(72)92402-6. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Epstein-Barr virus binding sites on lymphocyte subpopulations and the origin of lymphoblasts in cultured lymphoic cell lines and in the blood of patients with infectious mononucleosis. Clin Immunol Immunopathol. 1975 Mar;3(4):514–524. doi: 10.1016/0090-1229(75)90076-8. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M. Antibody-dependent cellular cytotoxicity (ADCC) against Epstein-Barr virus-determined membrane antigens. I. Reactivity in sera from normal persons and from patients with acute infectious mononucleosis. Clin Exp Immunol. 1976 Jul;25(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Clifford P., Klein E., Stjernswärd J. Search for tumor-specific immune reactions in Burkitt lymphoma patients by the membrane immunofluorescence reaction. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1628–1635. doi: 10.1073/pnas.55.6.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Pope J. H. Assay of the infectivity of Epstein-Barr virus by transformation of human leucocytes in vitro. J Gen Virol. 1972 Nov;17(2):233–236. doi: 10.1099/0022-1317-17-2-233. [DOI] [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Gerber P. Selective transformation of B lymphocytes by E.B. virus. Lancet. 1973 Jul 14;2(7820):93–94. doi: 10.1016/s0140-6736(73)93286-8. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Scott W., Moss D. J. Cell relationships in transformation of human leukocytes by Epstein-Barr virus. Int J Cancer. 1974 Jul 15;14(1):122–129. doi: 10.1002/ijc.2910140115. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Epstein M. A., Crawford D. H. Absence of infectious Epstein-Barr virus in blood in acute infectious mononucleosis. Nature. 1975 Nov 20;258(5532):236–236. doi: 10.1038/258236a0. [DOI] [PubMed] [Google Scholar]
- Rickinson A. B., Jarvis J. E., Crawford D. H., Epstein M. A. Observations on the type of infection by Epstein-Barr virus in peripheral lymphoid cells of patients with infectious mononucleosis. Int J Cancer. 1974 Dec 15;14(6):704–715. doi: 10.1002/ijc.2910140603. [DOI] [PubMed] [Google Scholar]
- Royston I., Sullivan J. L., Periman P. O., Perlin E. Cell-mediated immunity to Epstein-Barr-virus-transformed lymphoblastoid cells in acute infectious mononucleosis. N Engl J Med. 1975 Dec 4;293(23):1159–1163. doi: 10.1056/NEJM197512042932301. [DOI] [PubMed] [Google Scholar]
- Schneider U., zur Hausen H. Epstein-Barr virus-induced transformation of human leukocytes after cell fractionation. Int J Cancer. 1975 Jan 15;15(1):59–66. doi: 10.1002/ijc.2910150108. [DOI] [PubMed] [Google Scholar]
- Sheldon P. J., Hemsted E. H., Papamichail M., Holborow E. J. Thymic origin of atypical lymphoid cells in infectious mononucleosis. Lancet. 1973 May 26;1(7813):1153–1155. doi: 10.1016/s0140-6736(73)91148-3. [DOI] [PubMed] [Google Scholar]
- Svedmyr E. A., Deinhardt F., Klein G. Sensitivity of different target cells to the killing action of peripheral lymphocytes stimulated by autologous lymphoblastoid cell lines. Int J Cancer. 1974 Jun 15;13(6):891–903. doi: 10.1002/ijc.2910130617. [DOI] [PubMed] [Google Scholar]
- Svedmyr E., Jondal M. Cytotoxic effector cells specific for B Cell lines transformed by Epstein-Barr virus are present in patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1622–1626. doi: 10.1073/pnas.72.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedmyr E. Long-term maintenance in vitro of human T cells by repeated exposure to the same stimulator cells. Differences when using repeated stimulation in allogeneic mixed leukocyte culture and when using stimulation with autologous lymphoblastoid cells. Scand J Immunol. 1975 Sep;4(5-6):421–427. doi: 10.1111/j.1365-3083.1975.tb02647.x. [DOI] [PubMed] [Google Scholar]
- Virolainen M., Andersson L. C., Lalla M., von Essen R. T-lymphocyte proliferation in mononucleosis. Clin Immunol Immunopathol. 1973 Nov;2(1):114–120. doi: 10.1016/0090-1229(73)90041-x. [DOI] [PubMed] [Google Scholar]
- Wybran J., Chantler S., Fudenberg H. H. Isolation of normal T cells in chronic lymphatic leukaemia. Lancet. 1973 Jan 20;1(7795):126–129. doi: 10.1016/s0140-6736(73)90196-7. [DOI] [PubMed] [Google Scholar]
- Yata J., Desgranges C., de Thé G., Tachibana T. Lymphocytes in infectious mononucleosis. Properties of atypical cells and origin on the lymphoblastoid lines. Biomedicine. 1973 Nov 20;19(11):479–483. [PubMed] [Google Scholar]


