Abstract
Bone marrow cells were obtained from a patient with gamma heavy chain disease (HCD) whose serum contained a deleted immunoglobulin heavy chain. Incubation of the marrow cells with radioactive amino acids in short term tissue culture resulted in the synthesis of the labelled HCD protein. A permanent cell line was established from the peripheral blood of the patient. Similar labelling studies with the cell line and its cloned progeny demonstrated the synthesis of a protein identical in size and antigenicity to that synthesized by the marrow cells and found in the patient's serum. These experiments clearly demonstrated that, in this case of heavy chain disease, the deleted protein was the synthetic product of a clone of malignant lymphoid cells.
Full text
PDF

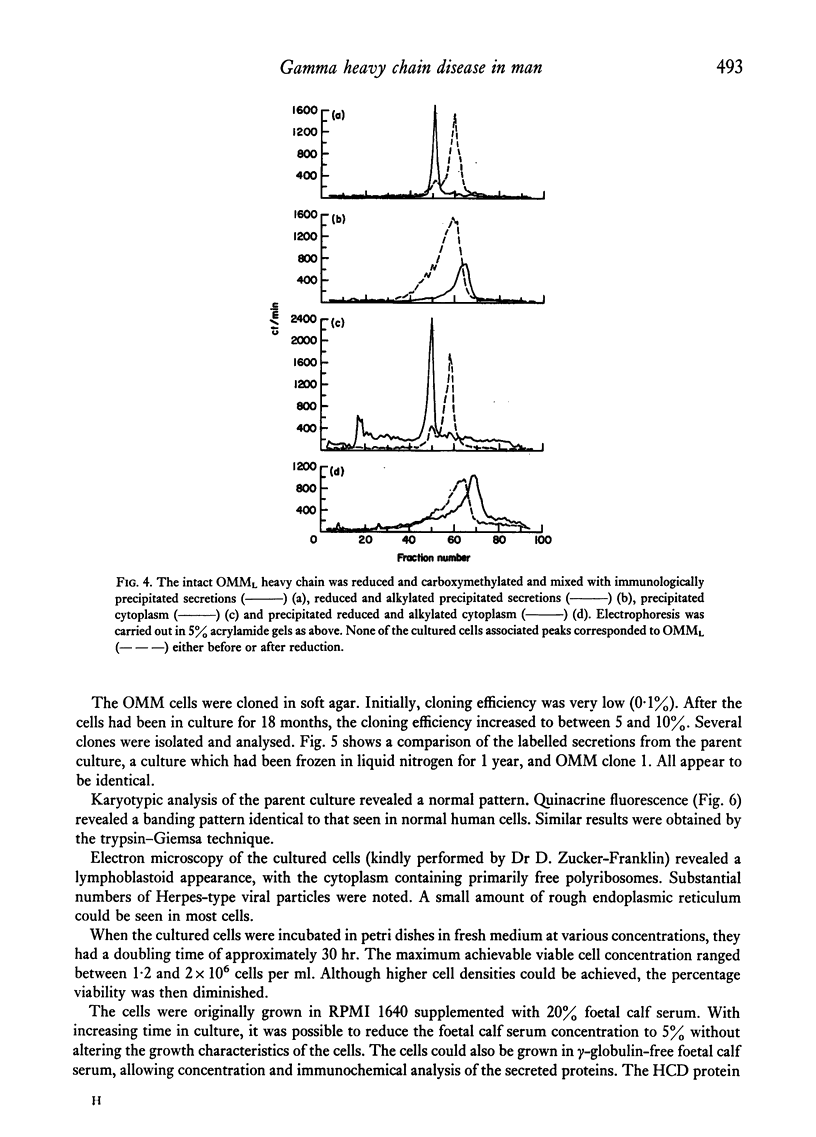
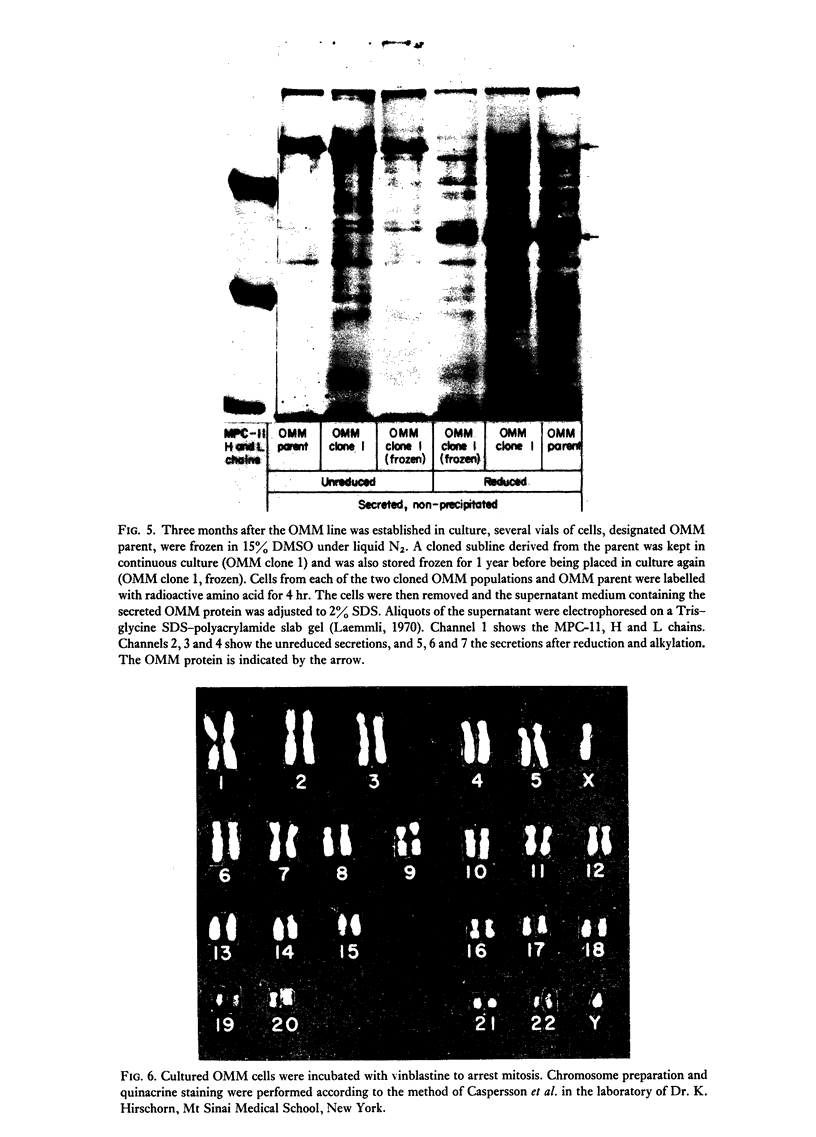



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlersberg J. B., Franklin E. C., Frangione B. Repetitive hinge region sequences in human IgG3: isolation of an 11,000-dalton fragment. Proc Natl Acad Sci U S A. 1975 Feb;72(2):723–727. doi: 10.1073/pnas.72.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlersberg J. B., Grann V., Zucker-Franklin D., Frangione B., Franklin E. C. An unusual case of a plasma cell neoplasm with an IgG3lambda myeloma and a gamma3 heavy chain disease protein. Blood. 1978 Jan;51(1):85–96. [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Preparation of mammalian polyribosomes with the detergent Nonidet P-40. Biochim Biophys Acta. 1967 Nov 21;149(1):302–304. doi: 10.1016/0005-2787(67)90715-0. [DOI] [PubMed] [Google Scholar]
- Buxbaum J. N., Preud'homme J. L. Alpha and gamma heavy chain diseases in man: intracellular origin of the aberrant polypeptides. J Immunol. 1972 Nov;109(5):1131–1137. [PubMed] [Google Scholar]
- Buxbaum J., Franklin E. C., Scharff M. D. Immunoglobulin M heavy chain disease: intracellular origin of the mu chain fragment. Science. 1970 Aug 21;169(3947):770–773. doi: 10.1126/science.169.3947.770. [DOI] [PubMed] [Google Scholar]
- Coffino P., Laskov R., Scharff M. D. Immunoglobulin production: method for quantitatively detecting variant myeloma cells. Science. 1970 Jan 9;167(3915):186–188. doi: 10.1126/science.167.3915.186. [DOI] [PubMed] [Google Scholar]
- Ein D., Buell D. N., Fahey J. L. Biosynthetic and structural studies of a heavy chain disease protein. J Clin Invest. 1969 Apr;48(4):785–793. doi: 10.1172/JCI106036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN E. C. STRUCTURAL STUDIES OF HUMAN 7S GAMMA-GLOBULIN (G IMMUNOGLOBULIN). FURTHER OBSERVATIONS OF A NATURALLY OCCURRING PROTEIN RELATED TO THE CRYSTALLIZABLE (FAST) FRAGMENT. J Exp Med. 1964 Nov 1;120:691–709. doi: 10.1084/jem.120.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Maizel J. V., Jr Molecular weight estimation of polypeptides by SDS-polyacrylamide gel electrophoresis: further data concerning resolving power and general considerations. Anal Biochem. 1969 Jun;29(3):505–514. doi: 10.1016/0003-2697(69)90335-2. [DOI] [PubMed] [Google Scholar]
- Usui M., Nariuchi H., Matuhasi T., Takahashi R., Adachihara K. A case of Fe-fragment disease. Jpn J Exp Med. 1971 Feb;41(1):83–87. [PubMed] [Google Scholar]
- Zolla S., Buxbaum J., Franklin E. C., Scharff M. D. Synthesis and assembly of immunoglobulins by malignant human plasmacytes. I. Myelomas producing gamma-chains and light chains. J Exp Med. 1970 Jul 1;132(1):148–162. doi: 10.1084/jem.132.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]