Abstract
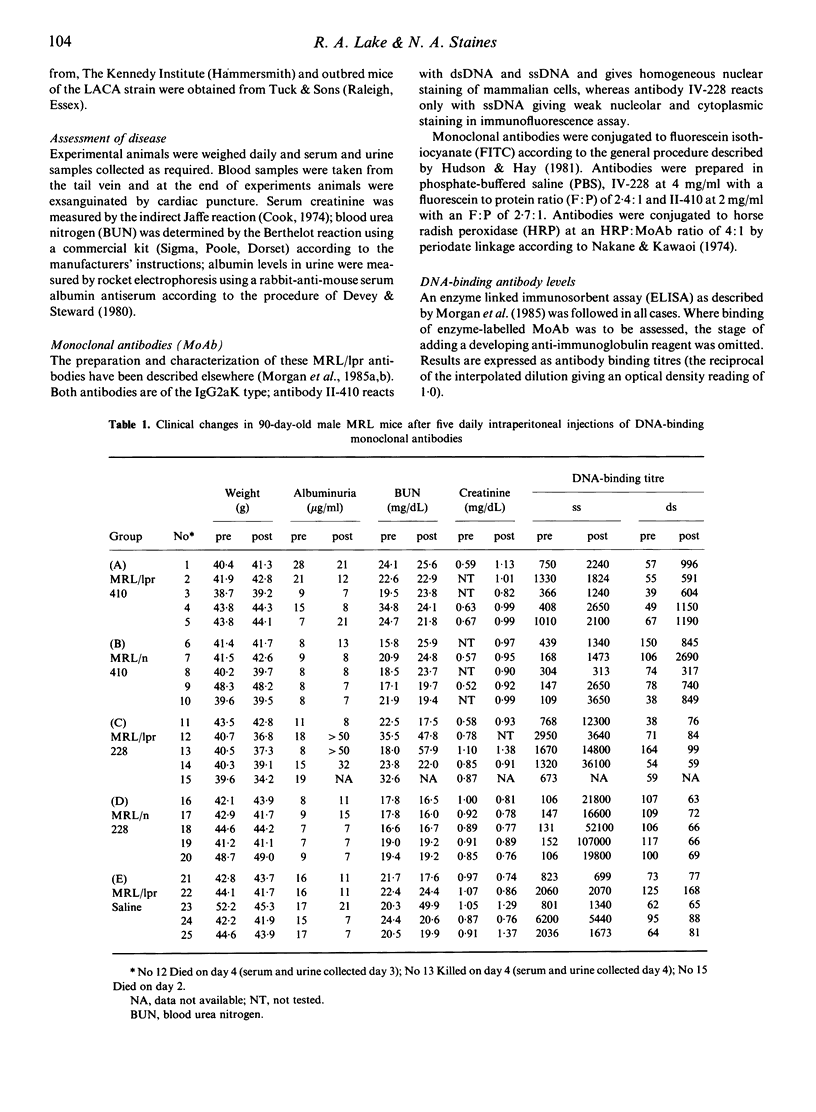
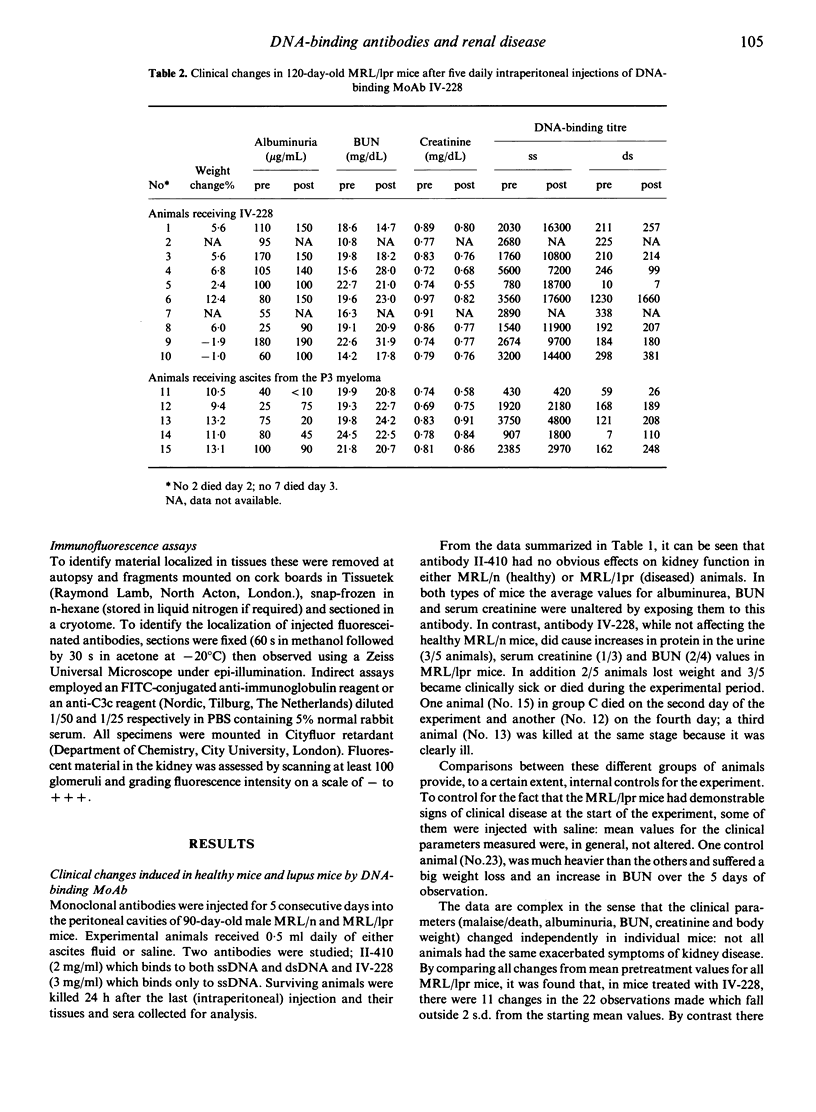
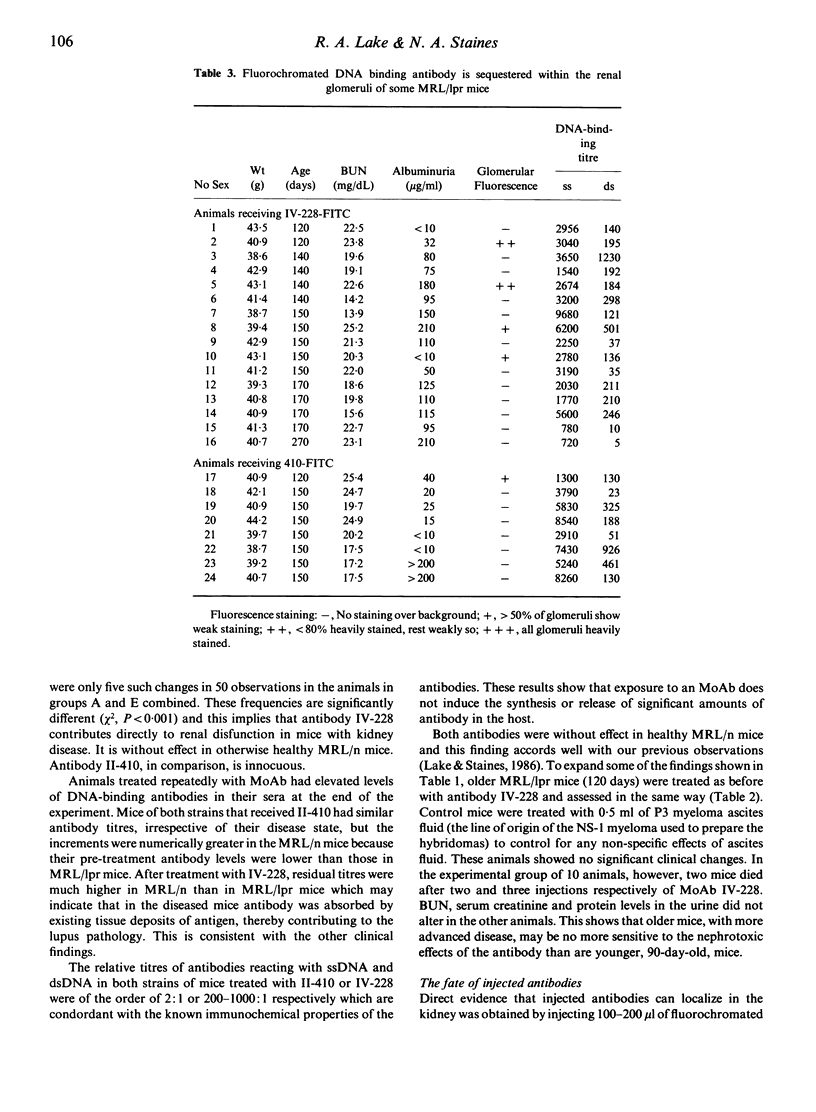
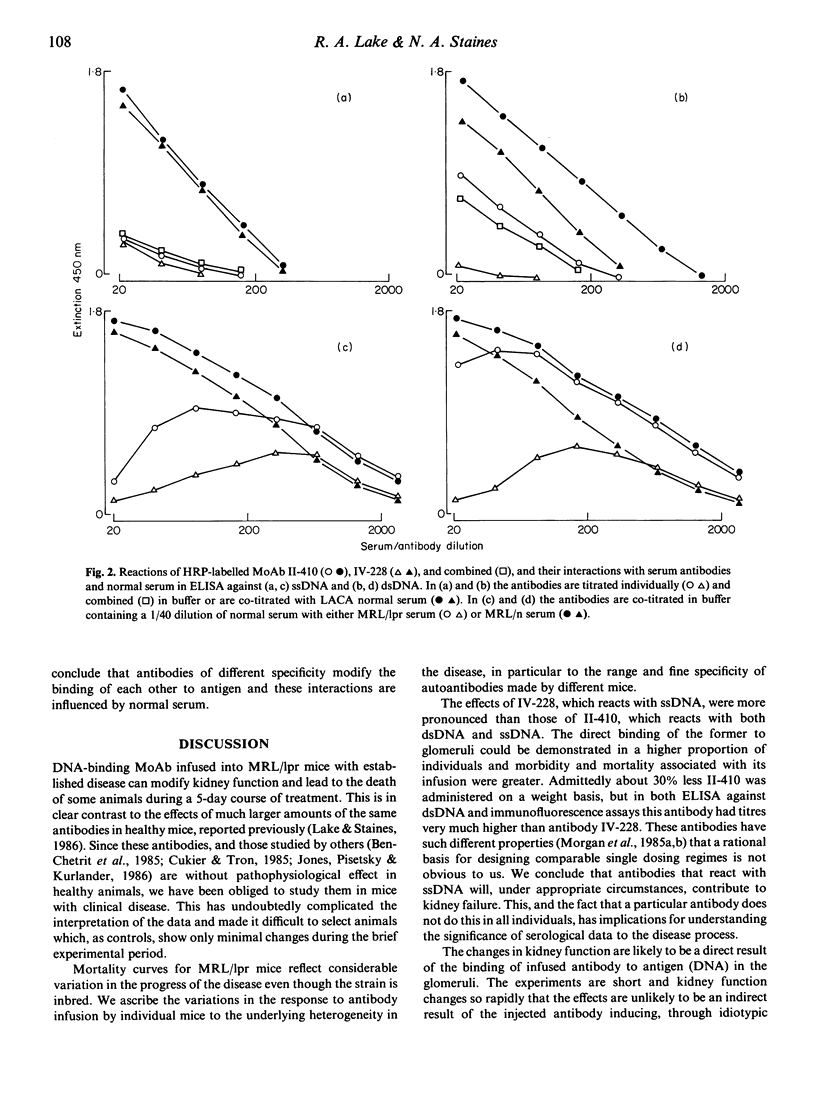
Two DNA-binding monoclonal antibodies (MoAb) derived from lupus mice were examined for their effects on kidney function. Antibody IV-228, reactive with single-stranded DNA (ssDNA) but not double-stranded DNA (dsDNA) significantly altered kidney function in some MRL/Mp-lpr/lpr (MRL/lpr) mice with lupus glomerulonephritis. Antibody II-410, preferentially reactive with dsDNA, did not modify kidney function in MRL/lpr mice. Neither antibody affected normal healthy MRL/Mp- +/+ (MRL/n) mice. Not all MRL/lpr mice were equally affected by IV-228 and in some animals with clinical disease it was without effect even when high circulating antibody levels were maintained by five sequential daily injections. Its affects upon kidney function appeared to be related to its ability to localize in vivo in glomeruli. It is assumed that epitopes on trapped immune complexes are immunologically available to circulating antibodies, and in those animals where injected antibody is not captured this is because the antigen epitope is either absent or is masked by the animals' own auto-antibodies. We conclude that antibodies which bind to ssDNA contribute to lupus nephritis; that individual mice make DNA-binding antibodies of different fine specificity; and that kidney disease is not always due to the same balance of antibodies in members of a genetically homogeneous stock.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben Chetrit E., Dunsky E. H., Wollner S., Eilat D. In vivo clearance and tissue uptake of an anti-DNA monoclonal antibody and its complexes with DNA. Clin Exp Immunol. 1985 Apr;60(1):159–168. [PMC free article] [PubMed] [Google Scholar]
- Cukier R., Tron F. Monoclonal anti-DNA antibodies: an approach to studying SLE nephritis. Clin Exp Immunol. 1985 Oct;62(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- Dang H., Harbeck R. J. The in vivo and in vitro glomerular deposition of isolated anti-double-stranded-DNA antibodies in NZB/W mice. Clin Immunol Immunopathol. 1984 Feb;30(2):265–278. doi: 10.1016/0090-1229(84)90061-8. [DOI] [PubMed] [Google Scholar]
- Devey M. E., Steward M. W. The induction of chronic antigen-antibody complex disease in selectively bred mice producing either high or low affinity antibody to protein antigens. Immunology. 1980 Oct;41(2):303–311. [PMC free article] [PubMed] [Google Scholar]
- Diamond B., Scharff M. D. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilat D., Hochberg M., Pumphrey J., Rudikoff S. Monoclonal antibodies to DNA and RNA from NZB/NZW F1 mice: antigenic specificities and NH2 terminal amino acid sequences. J Immunol. 1984 Jul;133(1):489–494. [PubMed] [Google Scholar]
- Hahn B. H., Ebling F. M. A public idiotypic determinant is present on spontaneous cationic IgG antibodies to DNA from mice of unrelated lupus-prone strains. J Immunol. 1984 Dec;133(6):3015–3019. [PubMed] [Google Scholar]
- Hahn B. H., Ebling F. M. Suppression of NZB/NZW murine nephritis by administration of a syngeneic monoclonal antibody to DNA. Possible role of anti-idiotypic antibodies. J Clin Invest. 1983 Jun;71(6):1728–1736. doi: 10.1172/JCI110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg D. A., Collins C. Detection of cross-reactive anti-DNA antibody idiotypes on renal tissue-bound immunoglobulins from lupus patients. J Clin Invest. 1985 Jul;76(1):287–294. doi: 10.1172/JCI111959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., Lambert P. H., Miescher P. A. In vitro demonstration of a particular affinity of glomerular basement membrane and collagen for DNA. A possible basis for a local formation of DNA-anti-DNA complexes in systemic lupus erythematosus. J Exp Med. 1976 Aug 1;144(2):428–443. doi: 10.1084/jem.144.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones F. S., Pisetsky D. S., Kurlander R. J. The clearance of a monoclonal anti-DNA antibody following administration of DNA in normal and autoimmune mice. Clin Immunol Immunopathol. 1986 Apr;39(1):49–60. doi: 10.1016/0090-1229(86)90204-7. [DOI] [PubMed] [Google Scholar]
- Koffler D., Schur P. H., Kunkel H. G. Immunological studies concerning the nephritis of systemic lupus erythematosus. J Exp Med. 1967 Oct 1;126(4):607–624. doi: 10.1084/jem.126.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler R., Perlmutter R. M., Noonan D. J., Dixon F. J., Theofilopoulos A. N. Ig heavy chain variable region gene complex of lupus mice exhibits normal restriction fragment length polymorphism. J Exp Med. 1985 Jul 1;162(1):346–351. doi: 10.1084/jem.162.1.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kávai M., Csipö I., Sonkoly I., Csongor J., Szegedi G. Y. Defective immune complex degradation by monocytes in patients with systemic lupus erythematosus. Scand J Immunol. 1986 Nov;24(5):527–532. doi: 10.1111/j.1365-3083.1986.tb02167.x. [DOI] [PubMed] [Google Scholar]
- Lake R. A., Morgan A., Henderson B., Staines N. A. A key role for fibronectin in the sequential binding of native dsDNA and monoclonal anti-DNA antibodies to components of the extracellular matrix: its possible significance in glomerulonephritis. Immunology. 1985 Feb;54(2):389–395. [PMC free article] [PubMed] [Google Scholar]
- Migliorini P., Ardman B., Kaburaki J., Schwartz R. S. Parallel sets of autoantibodies in MRL-lpr/lpr mice. An anti-DNA, anti-SmRNP, anti-gp70 network. J Exp Med. 1987 Feb 1;165(2):483–499. doi: 10.1084/jem.165.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A., Buchanan R. R., Lew A. M., Olsen I., Staines N. A. Five groups of antigenic determinants on DNA identified by monoclonal antibodies from (NZB X NZW)F1 and MRL/Mp-lpr/lpr mice. Immunology. 1985 May;55(1):75–83. [PMC free article] [PubMed] [Google Scholar]
- Morgan A., Isenberg D. A., Naparstek Y., Rauch J., Duggan D., Khiroya R., Staines N. A., Schattner A. Shared idiotypes are expressed on mouse and human anti-DNA autoantibodies. Immunology. 1985 Nov;56(3):393–399. [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., André-Schwartz J., Manser T., Wysocki L. J., Breitman L., Stollar B. D., Gefter M., Schwartz R. S. A single germline VH gene segment of normal A/J mice encodes autoantibodies characteristic of systemic lupus erythematosus. J Exp Med. 1986 Aug 1;164(2):614–626. doi: 10.1084/jem.164.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M. H., Gall D. G. Rat jejunal mucosal response to histamine and anti-histamines in vitro. Comparison with antigen-induced changes during intestinal anaphylaxis. Agents Actions. 1986 Oct;19(1-2):5–9. doi: 10.1007/BF01977249. [DOI] [PubMed] [Google Scholar]
- Roberts P. J., Isenberg D. A., Segal A. W. Defective degradation of bacterial DNA by phagocytes from patients with systemic and discoid lupus erythematosus. Clin Exp Immunol. 1987 Jul;69(1):68–78. [PMC free article] [PubMed] [Google Scholar]


