Abstract
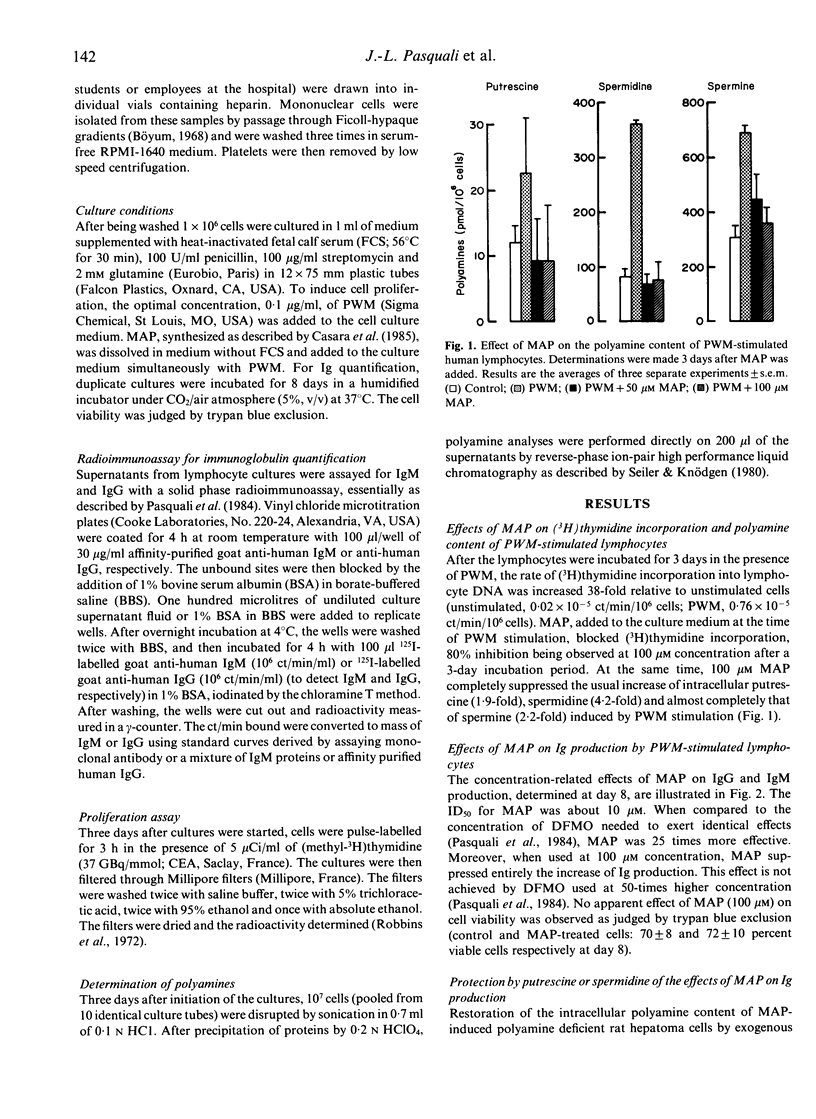
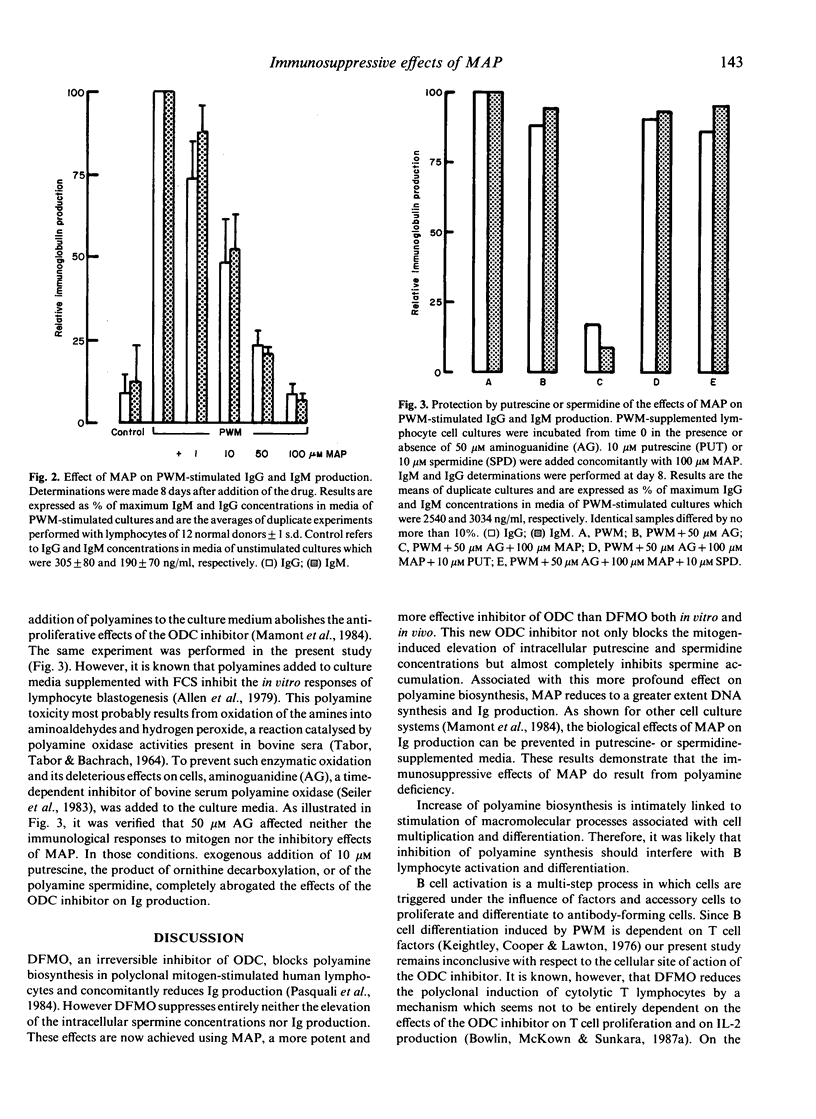
The consequences of specific inhibition of polyamine biosynthesis by (2R,5R)-6-heptyne-2,5-diamine (MAP) a potent inhibitor of L-ornithine decarboxylase (ODC), on immunoglobulin (Ig) production were studied in cultured human peripheral blood lymphocytes stimulated with pokeweed mitogen (PWM). MAP inhibits the usual PWM-induced increase of polyamine (putrescine, spermidine and spermine) concentrations and reduces concomitantly cell replication. In parallel with these biochemical effects, IgG and IgM production are diminished, a 95% decrease being observed at 100 microM MAP concentration. That the suppressive effects of the ODC inhibitor result from polyamine deficiency, and not from unrelated pharmacological effects, is demonstrated by the restoration of normal Ig production when 10 microM putrescine or spermidine are added to the culture medium. These findings established that the cellular immunological response can be affected by specific inhibition of polyamine biosynthesis and deserve further consideration both under in vitro and in vivo conditions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. C., Smith C. J., Hussain J. I., Thomas J. M., Gaugas J. M. Inhibition of lymphocyte proliferation by polyamines requires ruminant-plasma polyamine oxidase. Eur J Biochem. 1979 Dec;102(1):153–158. doi: 10.1111/j.1432-1033.1979.tb06275.x. [DOI] [PubMed] [Google Scholar]
- Bartholeyns J., Mamont P., Casara P. Antitumor properties of (2R,5R)-6-heptyne-2,5-diamine, a new potent enzyme-activated irreversible inhibitor of ornithine decarboxylase, in rodents. Cancer Res. 1984 Nov;44(11):4972–4977. [PubMed] [Google Scholar]
- Bowlin T. L., McKown B. J., Babcock G. F., Sunkara P. S. Intracellular polyamine biosynthesis is required for interleukin 2 responsiveness during lymphocyte mitogenesis. Cell Immunol. 1987 May;106(2):420–427. doi: 10.1016/0008-8749(87)90184-5. [DOI] [PubMed] [Google Scholar]
- Bowlin T. L., McKown B. J., Sunkara P. S. Increased ornithine decarboxylase activity and polyamine biosynthesis are required for optimal cytolytic T lymphocyte induction. Cell Immunol. 1987 Mar;105(1):110–117. doi: 10.1016/0008-8749(87)90060-8. [DOI] [PubMed] [Google Scholar]
- Bowlin T. L., McKown B. J., Sunkara P. S. Ornithine decarboxylase induction and polyamine biosynthesis are required for the growth of interleukin-2- and interleukin-3-dependent cell lines. Cell Immunol. 1986 Apr 1;98(2):341–350. doi: 10.1016/0008-8749(86)90294-7. [DOI] [PubMed] [Google Scholar]
- Böyum A. Separation of leukocytes from blood and bone marrow. Introduction. Scand J Clin Lab Invest Suppl. 1968;97:7–7. [PubMed] [Google Scholar]
- Danzin C., Casara P., Claverie N., Metcalf B. W., Jung M. J. (2R,5R)-6-heptyne-2,5-diamine, an extremely potent inhibitor of mammalian ornithine decarboxylase. Biochem Biophys Res Commun. 1983 Oct 14;116(1):237–243. doi: 10.1016/0006-291x(83)90406-0. [DOI] [PubMed] [Google Scholar]
- Ehrke M. J., Porter C. W., Eppolito C., Mihich E. Selective modulation by alpha-difluoromethylornithine of T-lymphocyte and antibody-mediated cytotoxic responses to mouse tumor allografts. Cancer Res. 1986 Jun;46(6):2798–2803. [PubMed] [Google Scholar]
- Fidelus R. K., Laughter A. H., Twomey J. J. The role of mitogens and lymphokines in the induction of ornithine decarboxylase (ODC) in T lymphocytes. J Immunol. 1984 Mar;132(3):1462–1465. [PubMed] [Google Scholar]
- Fillingame R. H., Jorstad C. M., Morris D. R. Increased cellular levels of spermidine or spermine are required for optimal DNA synthesis in lymphocytes activated by concanavalin A. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4042–4045. doi: 10.1073/pnas.72.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E., Jänne J., Hovi T. Suppression of the formation of polyamines and macromolecules by DL-alpha-difluoromethylornithine and methylglyoxal bis(guanylhydrazone) in phytohaemagglutinin-activated human lymphocytes. Biochem J. 1979 Jan 15;178(1):109–117. doi: 10.1042/bj1780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J. E., Lindsay V. J. Polyamine synthesis during lymphocyte activation. Induction of ornithine decarboxylase and S-adenosyl methionine decarboxylase. Exp Cell Res. 1973 Mar 15;77(1):428–436. doi: 10.1016/0014-4827(73)90597-1. [DOI] [PubMed] [Google Scholar]
- Keightley R. G., Cooper M. D., Lawton A. R. The T cell dependence of B cell differentiation induced by pokeweed mitogen. J Immunol. 1976 Nov;117(5 Pt 1):1538–1544. [PubMed] [Google Scholar]
- Mamont P. S., Siat M., Joder-Ohlenbusch A. M., Bernhardt A., Casara P. Effects of (2R, 5R)-6-heptyne-2,5-diamine, a potent inhibitor of L-ornithine decarboxylase, on rat hepatoma cells cultured in vitro. Eur J Biochem. 1984 Aug 1;142(3):457–463. doi: 10.1111/j.1432-1033.1984.tb08308.x. [DOI] [PubMed] [Google Scholar]
- Melchers F., Andersson J. Factors controlling the B-cell cycle. Annu Rev Immunol. 1986;4:13–36. doi: 10.1146/annurev.iy.04.040186.000305. [DOI] [PubMed] [Google Scholar]
- Pasquali J. L., Urlacher A., Weryha A., Storck D., Mamont P. S. Inhibition by DL-alpha-difluoromethylornithine of the pokeweed mitogen-induced immunoglobulin production in cultured human lymphocytes. Immunopharmacology. 1984 Jun;7(3-4):145–149. doi: 10.1016/0162-3109(84)90030-4. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. H., Gart J. J., Levis W. R., Burk P. G. The millipore filter assay technique for measuring tritiated thymidine incorporation into DNA in leucocyte cultures. Clin Exp Immunol. 1972 Aug;11(4):629–640. [PMC free article] [PubMed] [Google Scholar]
- Seiler N., Knödgen B. High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr. 1980 Dec 12;221(2):227–235. doi: 10.1016/s0378-4347(00)84307-8. [DOI] [PubMed] [Google Scholar]
- Seyfried C. E., Morris D. R. Relationship between inhibition of polyamine biosynthesis and DNA replication in activated lymphocytes. Cancer Res. 1979 Dec;39(12):4861–4867. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., BACHRACH U. IDENTIFICATION OF THE AMINOALDEHYDES PRODUCED BY THE OXIDATION OF SPERMINE AND SPERMIDINE WITH PURIFIED PLASMA AMINE OXIDASE. J Biol Chem. 1964 Jul;239:2194–2203. [PubMed] [Google Scholar]
- Watanabe T., Kishimoto T., Miyake T., Nishizawa Y., Inoue H. Triggering mechanism of B lymphocytes. II. Induction of ornithine decarboxylase in B cells by anti-immunoglobulin and enhancing soluble factor. J Immunol. 1975 Nov;115(5):1185–1190. [PubMed] [Google Scholar]


