Abstract
Autoimmune-prone mice homozygous for the lpr gene develop prominent lymphadenopathy composed mainly of Thy-1+ CD8- CD4- B220+ cells. Expression patterns of B220 vs CD4 on lymph node cells from lpr mice were analysed using two-colour flow microfluorometry. B220+CD4+ cells, which were hardly seen in lymph nodes of B6-+/+ mice, increased significantly in B6-lpr mice with ageing. Functional analysis of purified B220+ CD4+ cells from lpr mice revealed that these cells scarcely responded to T cell mitogens with or without rIL-2. Furthermore, B220+ CD4+ cells were defective in IL-2 production when cultured with Con A. On the other hand, B220-CD4+ cells from B6-lpr mice showed an ability to respond to T cell mitogens similar to that of B220- CD4+ cells from B6-+/+ mice. These results indicate that an unusual T cell subset expressing both B220 and CD4 in lpr mice is functionally defective, but the intrinsic ability of B220-CD4+ cells is almost intact as compared with the counterpart in normal mice.
Full text
PDF
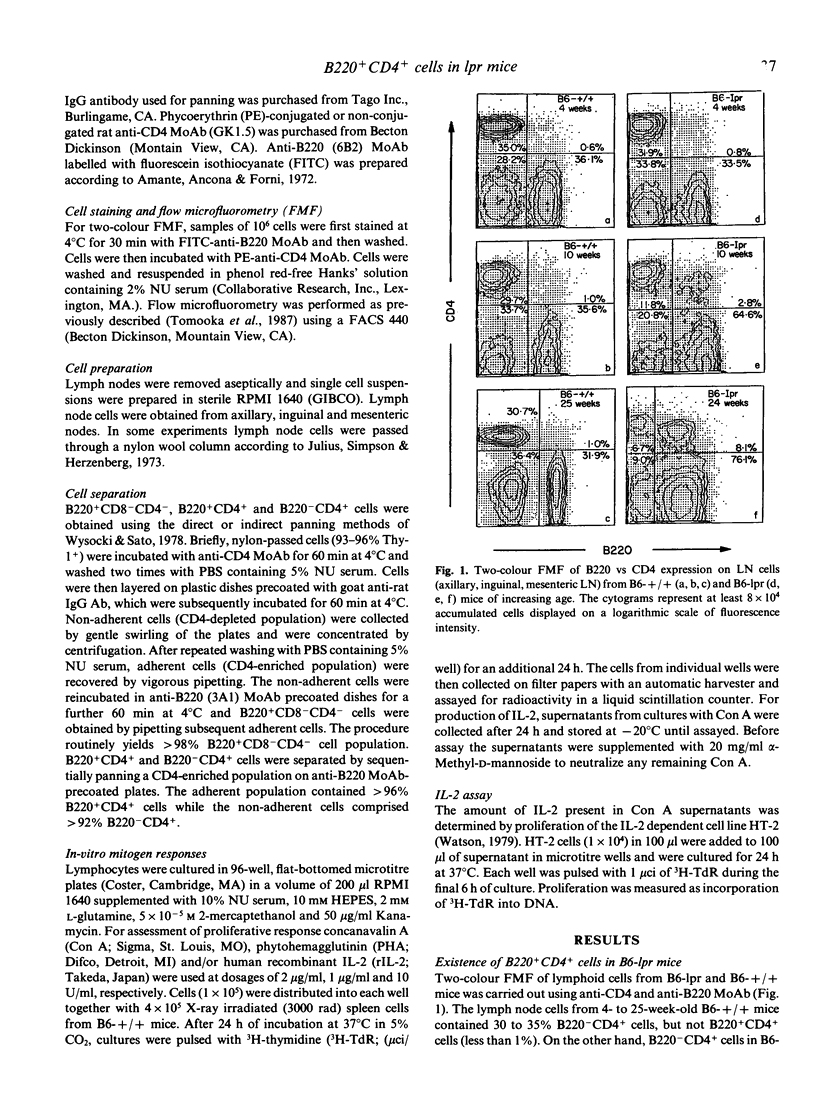
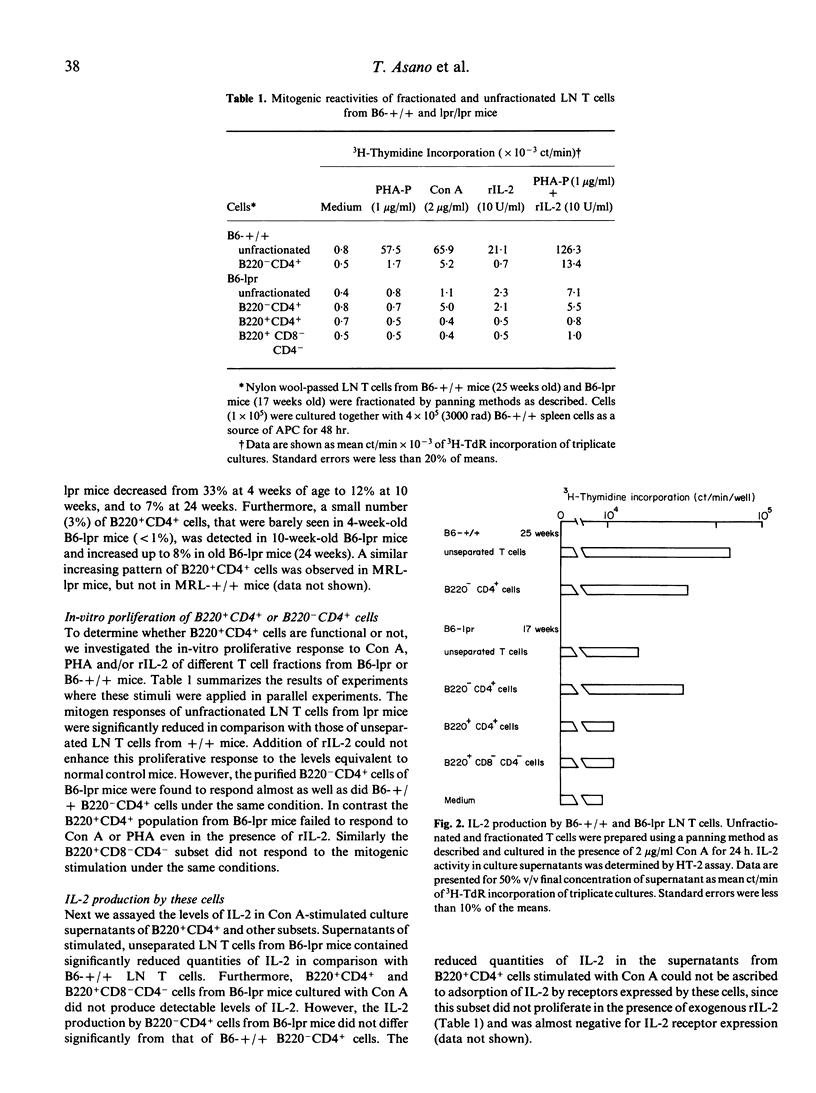


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amante L., Ancona A., Forni L. The conjugation of immunoglobulins with tetramethylrhodamine isothiocyanate. A comparison between the amorphous and the crystalline fluorochrome. J Immunol Methods. 1972 May;1(3):289–301. doi: 10.1016/0022-1759(72)90006-3. [DOI] [PubMed] [Google Scholar]
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. A monoclonal antibody that recognizes B cells and B cell precursors in mice. J Exp Med. 1981 Feb 1;153(2):269–279. doi: 10.1084/jem.153.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W. F., Dumont F. J., Bedigian H. G., Fowlkes B. J., Morse H. C., 3rd Phenotypic, functional, and molecular genetic comparisons of the abnormal lymphoid cells of C3H-lpr/lpr and C3H-gld/gld mice. J Immunol. 1986 Jun 1;136(11):4075–4084. [PubMed] [Google Scholar]
- Davignon J. L., Budd R. C., Ceredig R., Piguet P. F., MacDonald H. R., Cerottini J. C., Vassalli P., Izui S. Functional analysis of T cell subsets from mice bearing the lpr gene. J Immunol. 1985 Oct;135(4):2423–2428. [PubMed] [Google Scholar]
- Fischbach M. Defective T cell response to presented antigen in autoimmune mice. J Immunol. 1984 Nov;133(5):2365–2368. [PubMed] [Google Scholar]
- Hefeneider S. H., Conlon P. J., Dower S. K., Henney C. S., Gillis S. Limiting dilution analysis of interleukin 2 and colony-stimulating factor producer cells in normal and autoimmune mice. J Immunol. 1984 Apr;132(4):1863–1868. [PubMed] [Google Scholar]
- Izui S., Kelley V. E., Masuda K., Yoshida H., Roths J. B., Murphy E. D. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J Immunol. 1984 Jul;133(1):227–233. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Katagiri K., Katagiri T., Eisenberg R. A., Ting J., Cohen P. L. Interleukin 2 responses of lpr and normal L3T4-/Lyt-2- T cells induced by TPA plus A23187. J Immunol. 1987 Jan 1;138(1):149–156. [PubMed] [Google Scholar]
- Lewis D. E., Giorgi J. V., Warner N. L. Flow cytometry analysis of T cells and continuous T-cell lines from autoimmune MRL/l mice. Nature. 1981 Jan 22;289(5795):298–300. doi: 10.1038/289298a0. [DOI] [PubMed] [Google Scholar]
- Mage M., Mathieson B., Sharrow S., McHugh L., Hämmerling U., Kanellopoulos-Langevin C., Brideau D., Jr, Thomas C. A., 3rd Preparative nonlytic separation of Lyt2+ and Lyt2- T lymphocytes, functional analyses of the separated cells and demonstration of synergy in graft-vs.-host reaction of Lyt2+ and Lyt2- cells. Eur J Immunol. 1981 Mar;11(3):228–235. doi: 10.1002/eji.1830110312. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Davidson W. F., Yetter R. A., Murphy E. D., Roths J. B., Coffman R. L. Abnormalities induced by the mutant gene Ipr: expansion of a unique lymphocyte subset. J Immunol. 1982 Dec;129(6):2612–2615. [PubMed] [Google Scholar]
- Santoro T. J., Lehmann K. R., Batt R. A., Kotzin B. L. The role of L3T4+ cells in the pathogenesis of lupus in lpr-bearing mice. I. Defects in the production of interleukins 2 and 3. Eur J Immunol. 1987 Aug;17(8):1131–1136. doi: 10.1002/eji.1830170809. [DOI] [PubMed] [Google Scholar]
- Santoro T. J., Luger T. A., Ravache E. S., Smolen J. S., Oppenheim J. J., Steinberg A. D. In vitro correction of the interleukin 2 defect of autoimmune mice. Eur J Immunol. 1983 Jul;13(7):601–604. doi: 10.1002/eji.1830130717. [DOI] [PubMed] [Google Scholar]
- Tomooka S., Matsuzaki G., Kishihara K., Tanaka K., Yoshikai Y., Taniguchi K., Himeno K., Nomoto K. Sequential appearance of thymocyte subpopulations and T cell antigen receptor gene messages in the mouse thymus after sublethal irradiation. J Immunol. 1987 Dec 15;139(12):3986–3990. [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofsy D., Hardy R. R., Seaman W. E. The proliferating cells in autoimmune MRL/lpr mice lack L3T4, an antigen on "helper" T cells that is involved in the response to class II major histocompatibility antigens. J Immunol. 1984 Jun;132(6):2686–2689. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]


