Abstract
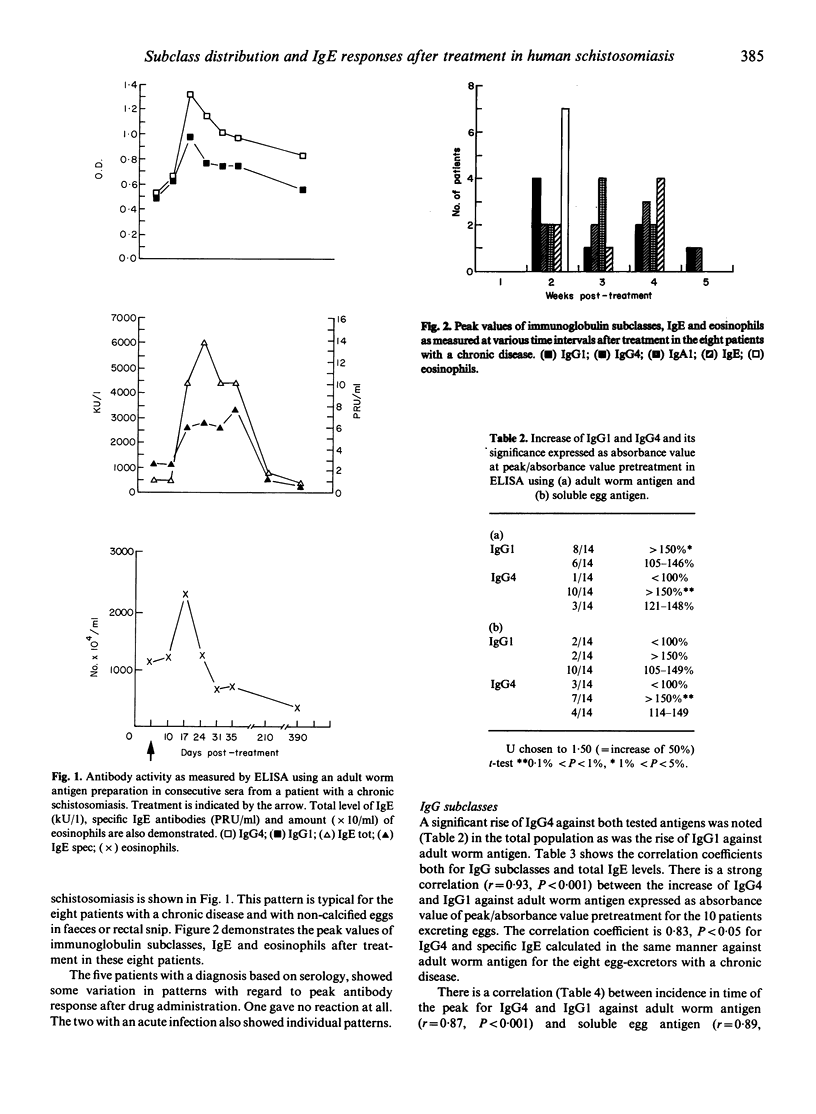
The IgG and IgA subclass distribution of specific antibodies as well as the distribution of total and specific IgE in 15 patients with schistosomiasis was determined in consecutive samples before and after initiation of treatment. An adult worm antigen preparation and a soluble egg antigen preparation were used as antigens in the ELISA assays. After initiation of treatment a rise was noted in certain subclasses and a correlation was found for specific IgG1 and IgG4 serum levels in the egg-excreting patients against adult worm antigen and for specific IgG4 and IgE levels in sera from the eight patients with a chronic disease. They also had a rise of the specific IgA1 titre and six of them also of specific IgA2. Members of eosinophilic granulocytes reached a peak after 2 weeks in seven of the eight patients. The increase of eosinophils was an early event as opposed to the incidence of peak of the determined specific isotypes. The associated rise in IgG1, IgG4 and IgE antibody concentrations and eosinophils may suggest a causal relation possibly induced by common interleukins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalberse R. C., van der Gaag R., van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983 Feb;130(2):722–726. [PubMed] [Google Scholar]
- Azuma C., Tanabe T., Konishi M., Kinashi T., Noma T., Matsuda F., Yaoita Y., Takatsu K., Hammarström L., Smith C. I. Cloning of cDNA for human T-cell replacing factor (interleukin-5) and comparison with the murine homologue. Nucleic Acids Res. 1986 Nov 25;14(22):9149–9158. doi: 10.1093/nar/14.22.9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P. Effector and regulatory mechanisms in immunity to schistosomes: a heuristic view. Annu Rev Immunol. 1985;3:455–476. doi: 10.1146/annurev.iy.03.040185.002323. [DOI] [PubMed] [Google Scholar]
- Catty D., Jassim A., Hassan K., Raykundalia C. IgG subclasses in parasitic infestations. Monogr Allergy. 1986;19:144–155. [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Flanagan J. G., Rabbitts T. H. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a segment containing gamma, epsilon and alpha genes. Nature. 1982 Dec 23;300(5894):709–713. doi: 10.1038/300709a0. [DOI] [PubMed] [Google Scholar]
- Hammarström L., Granström M., Oxelius V., Persson M. A., Smith C. I. IgG subclass distribution of antibodies against S. aureus teichoic acid and alpha-toxin in normal and immunodeficient donors. Clin Exp Immunol. 1984 Mar;55(3):593–601. [PMC free article] [PubMed] [Google Scholar]
- Hammarström L., Persson M. A., Smith C. I. Subclass distribution of human anti-Staphylococcus aureus alpha toxin antibodies: suggestion of an IgG1, IgA1, IgG4 switch pattern. Scand J Immunol. 1984 Sep;20(3):247–250. doi: 10.1111/j.1365-3083.1984.tb00998.x. [DOI] [PubMed] [Google Scholar]
- Hussain R., Ottesen E. A. IgE responses in human filariasis. IV. Parallel antigen recognition by IgE and IgG4 subclass antibodies. J Immunol. 1986 Mar 1;136(5):1859–1863. [PubMed] [Google Scholar]
- Iskander R., Das P. K., Aalberse R. C. IgG4 antibodies in Egyptian patients with schistosomiasis. Int Arch Allergy Appl Immunol. 1981;66(2):200–207. doi: 10.1159/000232819. [DOI] [PubMed] [Google Scholar]
- Jassim A., Hassan K., Catty D. Antibody isotypes in human schistosomiasis mansoni. Parasite Immunol. 1987 Nov;9(6):627–650. doi: 10.1111/j.1365-3024.1987.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Johansson S. G., Bennich H., Berg T. In vitro diagnosis of atopic allergy. 3. Quantitative estimation of circulating IgE antibodies by the radioallergosorbent test. Int Arch Allergy Appl Immunol. 1971;41(2):443–451. [PubMed] [Google Scholar]
- Khalife J., Capron M., Capron A., Grzych J. M., Butterworth A. E., Dunne D. W., Ouma J. H. Immunity in human schistosomiasis mansoni. Regulation of protective immune mechanisms by IgM blocking antibodies. J Exp Med. 1986 Nov 1;164(5):1626–1640. doi: 10.1084/jem.164.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun P., Spiegelberg H. L. Concomitant immunoglobulin E and immunoglobulin G1 formation in Nippostrongylus brasiliensis-infected mice. J Immunol. 1987 Sep 1;139(5):1459–1465. [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., Russell M. W. IgA subclasses. Monogr Allergy. 1986;19:277–301. [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Persson M. A., Hammarström L., Smith C. I. Enzyme-linked immunosorbent assay for subclass distribution of human IgG and IgA antigen-specific antibodies. J Immunol Methods. 1985 Apr 8;78(1):109–121. doi: 10.1016/0022-1759(85)90334-5. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., O'Garra A., Warren D. J., Klaus G. G. Eosinophil differentiation factor also has B-cell growth factor activity: proposed name interleukin 4. Proc Natl Acad Sci U S A. 1986 Jan;83(2):437–440. doi: 10.1073/pnas.83.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Warren D. J., Strath M. Identification of a lymphokine that stimulates eosinophil differentiation in vitro. Its relationship to interleukin 3, and functional properties of eosinophils produced in cultures. J Exp Med. 1985 Jul 1;162(1):60–74. doi: 10.1084/jem.162.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Rennick D. M. Characterization of a murine lymphokine distinct from interleukin 2 and interleukin 3 (IL-3) possessing a T-cell growth factor activity and a mast-cell growth factor activity that synergizes with IL-3. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1857–1861. doi: 10.1073/pnas.83.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundqvist V. A., Linde A., Wahren B. Virus-specific immunoglobulin G subclasses in herpes simplex and varicella-zoster virus infections. J Clin Microbiol. 1984 Jul;20(1):94–98. doi: 10.1128/jcm.20.1.94-98.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


