Abstract
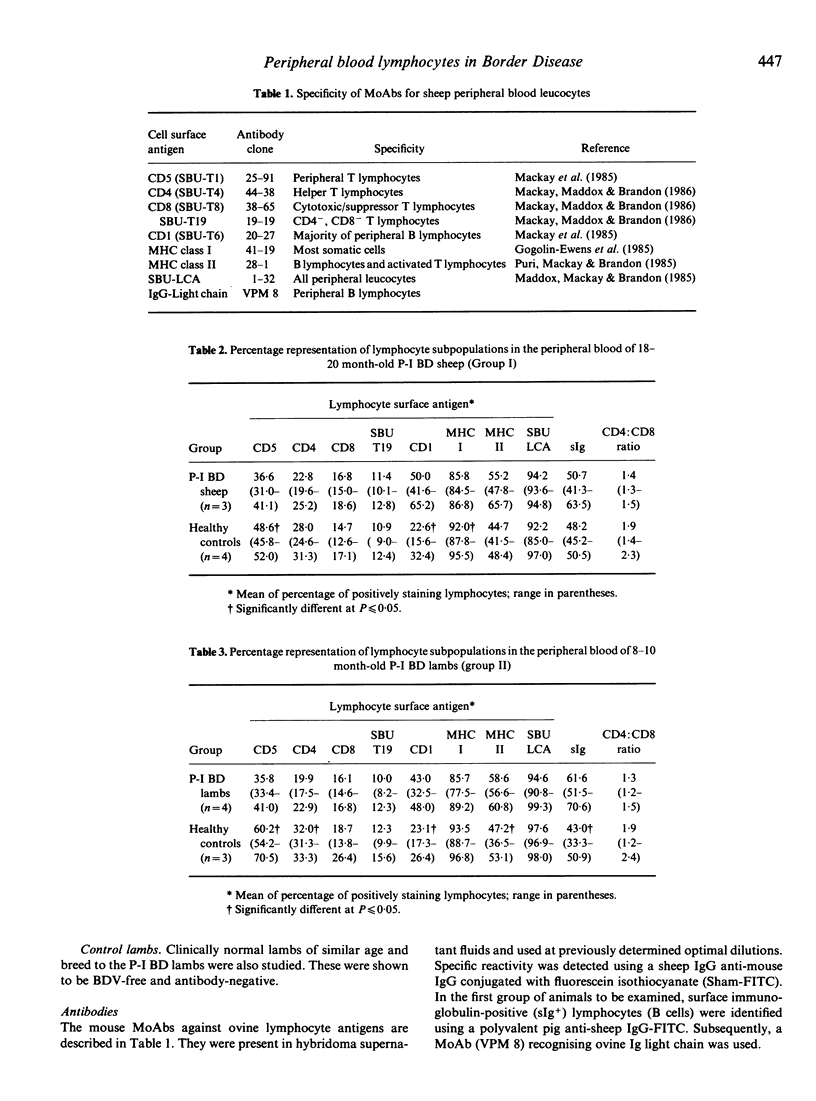
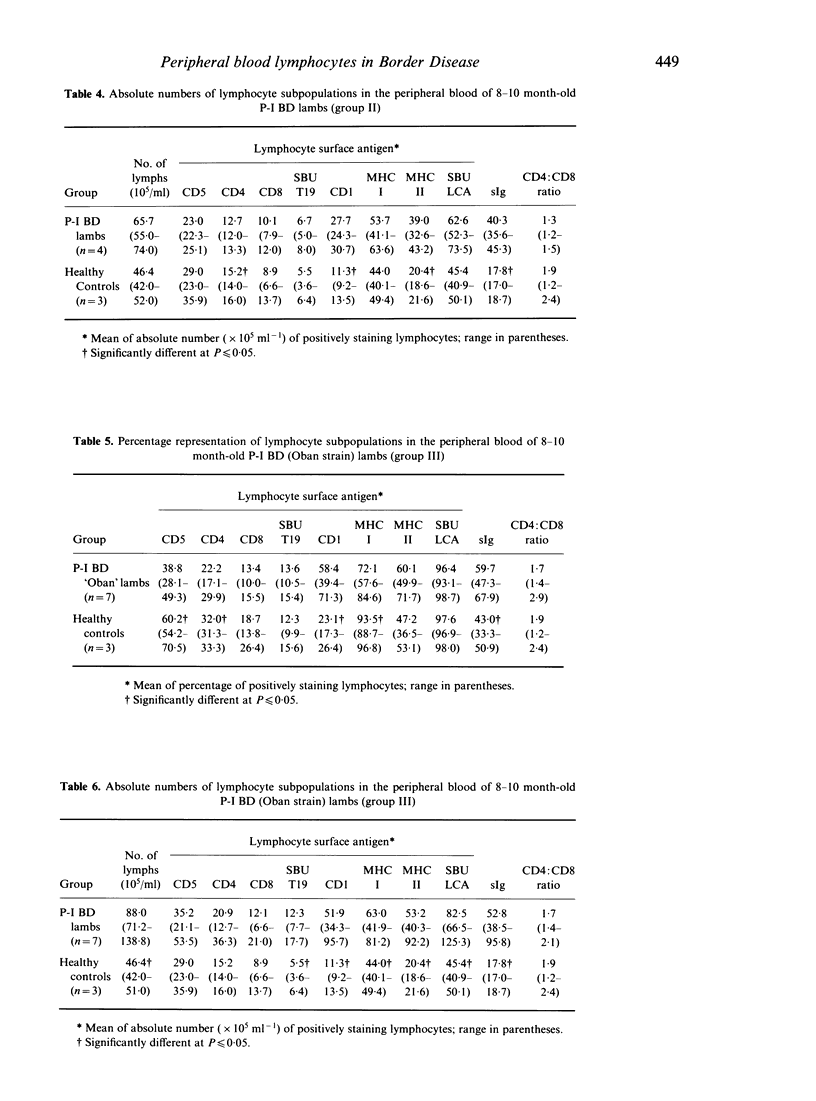
The surface phenotypes of peripheral blood lymphocytes in groups of lambs and adult sheep persistently infected with Border disease virus (P-I BD) were compared with those of healthy controls. The proportion and number of lymphocytes bearing surface immunoglobulin (sIg+) and expressing class II MHC antigen (B cells) were significantly increased. A significant increase in CD1+ lymphocytes was also evident. Conversely, the proportion of T lymphocytes in P-I BD lambs was reduced. A marked reduction in the proportion of circulating lymphocytes expressing class I MHC antigen was also observed. These findings were not affected by differences in the strain of the virus responsible for the persistent infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Barlow R. M. Experiments in border disease. IV. Pathological changes in ewes. J Comp Pathol. 1972 Apr;82(2):151–157. doi: 10.1016/0021-9975(72)90057-6. [DOI] [PubMed] [Google Scholar]
- Bielefeldt Ohmann H., Rønsholt L., Bloch B. Demonstration of bovine viral diarrhoea virus in peripheral blood mononuclear cells of persistently infected, clinically normal cattle. J Gen Virol. 1987 Jul;68(Pt 7):1971–1982. doi: 10.1099/0022-1317-68-7-1971. [DOI] [PubMed] [Google Scholar]
- Bolin S. R., McClurkin A. W., Coria M. F. Effects of bovine viral diarrhea virus on the percentages and absolute numbers of circulating B and T lymphocytes in cattle. Am J Vet Res. 1985 Apr;46(4):884–886. [PubMed] [Google Scholar]
- Bolin S. R., Sacks J. M., Crowder S. V. Frequency of association of noncytopathic bovine viral diarrhea virus with mononuclear leukocytes from persistently infected cattle. Am J Vet Res. 1987 Oct;48(10):1441–1445. [PubMed] [Google Scholar]
- Bonniwell M. A., Nettleton P. F., Gardiner A. C., Barlow R. M., Gilmour J. S. Border disease without nervous signs or fleece changes. Vet Rec. 1987 Mar 14;120(11):246–249. doi: 10.1136/vr.120.11.246. [DOI] [PubMed] [Google Scholar]
- Coria M. F., McClurkin A. W. Specific immune tolerance in an apparently healthy bull persistently infected with bovine viral diarrhea virus. J Am Vet Med Assoc. 1978 Feb 15;172(4):449–451. [PubMed] [Google Scholar]
- Gardiner A. C., Nettleton P. F., Barlow R. M. Virology and immunology of a spontaneous and experimental mucosal disease-like syndrome in sheep recovered from clinical border disease. J Comp Pathol. 1983 Jul;93(3):463–469. doi: 10.1016/0021-9975(83)90033-6. [DOI] [PubMed] [Google Scholar]
- Gogolin-Ewens K. J., Mackay C. R., Mercer W. R., Brandon M. R. Sheep lymphocyte antigens (OLA). I. Major histocompatibility complex class I molecules. Immunology. 1985 Dec;56(4):717–723. [PMC free article] [PubMed] [Google Scholar]
- Hadjisavvas T. H., Harkness J. W., Huck R. A., Stuart P. The demonstration by interference tests of an infective agent in fetuses from ewes inoculated with Border disease tissue. Res Vet Sci. 1975 May;18(3):237–243. [PubMed] [Google Scholar]
- Harkness J. W., King A. A., Terlecki S., Sands J. J. Border disease of sheep: isolation of the virus in tissue culture and experimental reproduction of the disease. Vet Rec. 1977 Jan 22;100(4):71–72. doi: 10.1136/vr.100.4.71. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Tanaka K., Jay F., Khoury G., Jay G. Modulation of the tumorigenicity of human adenovirus-12-transformed cells by interferon. Cell. 1985 Nov;43(1):263–267. doi: 10.1016/0092-8674(85)90031-5. [DOI] [PubMed] [Google Scholar]
- Jennings S. R., Rice P. L., Kloszewski E. D., Anderson R. W., Thompson D. L., Tevethia S. S. Effect of herpes simplex virus types 1 and 2 on surface expression of class I major histocompatibility complex antigens on infected cells. J Virol. 1985 Dec;56(3):757–766. doi: 10.1128/jvi.56.3.757-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. W., Muscoplat C. C. Immunologic abnormalities in calves with chronic bovine viral diarrhea. Am J Vet Res. 1973 Sep;34(9):1139–1141. [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Brandon M. R. Three distinct subpopulations of sheep T lymphocytes. Eur J Immunol. 1986 Jan;16(1):19–25. doi: 10.1002/eji.1830160105. [DOI] [PubMed] [Google Scholar]
- Mackay C. R., Maddox J. F., Gogolin-Ewens K. J., Brandon M. R. Characterization of two sheep lymphocyte differentiation antigens, SBU-T1 and SBU-T6. Immunology. 1985 Aug;55(4):729–737. [PMC free article] [PubMed] [Google Scholar]
- Maddox J. F., Mackay C. R., Brandon M. R. The sheep analogue of leucocyte common antigen (LCA). Immunology. 1985 Jun;55(2):347–353. [PMC free article] [PubMed] [Google Scholar]
- McClurkin A. W., Littledike E. T., Cutlip R. C., Frank G. H., Coria M. F., Bolin S. R. Production of cattle immunotolerant to bovine viral diarrhea virus. Can J Comp Med. 1984 Apr;48(2):156–161. [PMC free article] [PubMed] [Google Scholar]
- Mims C. A. Factors in the mechanism of persistence of viral infections. Prog Med Virol. 1974;18(0):1–14. [PubMed] [Google Scholar]
- Ohmann H. B., Jensen M. H., Sørensen K. J., Dalsgaard K. Experimental fetal infection with bovine viral diarrhea virus. I. Virological and serological studies. Can J Comp Med. 1982 Oct;46(4):357–362. [PMC free article] [PubMed] [Google Scholar]
- Puri N. K., Mackay C. R., Brandon M. R. Sheep lymphocyte antigens (OLA). II. Major histocompatibility complex class II molecules. Immunology. 1985 Dec;56(4):725–733. [PMC free article] [PubMed] [Google Scholar]
- Roth J. A., Bolin S. R., Frank D. E. Lymphocyte blastogenesis and neutrophil function in cattle persistently infected with bovine viral diarrhea virus. Am J Vet Res. 1986 May;47(5):1139–1141. [PubMed] [Google Scholar]
- Sawyer M. M., Schore C. E., Menzies P. I., Osburn B. I. Border disease in a flock of sheep: epidemiologic, laboratory, and clinical findings. J Am Vet Med Assoc. 1986 Jul 1;189(1):61–65. [PubMed] [Google Scholar]
- Steck F., Lazary S., Fey H., Wandeler A., Huggler C., Oppliger G., Baumberger H., Kaderli R., Martig J. Immune responsiveness in cattle fatally affected by bovine virus diarrhea-mucosal disease. Zentralbl Veterinarmed B. 1980;27(6):429–445. doi: 10.1111/j.1439-0450.1980.tb01790.x. [DOI] [PubMed] [Google Scholar]
- Terpstra C. Border disease: virus persistence, antibody response and transmission studies. Res Vet Sci. 1981 Mar;30(2):185–191. [PubMed] [Google Scholar]
- Terpstra C. Detection of Border disease antigen in tissues of affected sheep and in cell cultures by immunofluorescence. Res Vet Sci. 1978 Nov;25(3):350–355. [PubMed] [Google Scholar]
- Truitt R. L., Schechmeister I. L. The replication of bovine viral diarrhea-mucosal disease virus in bovine leukocytes in vitro. Arch Gesamte Virusforsch. 1973;42(1):78–87. doi: 10.1007/BF01250509. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]


