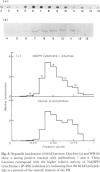
Abstract
Sera from 23 children with autoimmune chronic active hepatitis and positive for anti-liver-kidney-microsome antibody (LKMA), as defined by immunofluorescence, were analysed by Western blot (WB) and two-dimensional gel electrophoresis using rat liver microsomes as antigen, and by WB and dot-blot analysis with rat liver microsomal subfractions. Western blot analysis showed three patterns of reactivity: 13 sera recognized a 50 kD polypeptide, six sera a 66 kD polypeptide and four sera both of them. Two-dimensional gel electrophoresis, WB, and dot-blot analysis showed the 66 kD antigen to have a pI of 5.4 and to be located in the smooth domain of the endoplasmic reticulum. Western blot analysis using monospecific antisera against human IgG subclasses showed the LKMA directed against the 66 kD antigen to be mainly of the IgG1 subclass. These results indicate that LKMA associated with a subgroup of autoimmune hepatitis of children react with at least two different microsomal antigens in rat liver: (1) the 50 kD polypeptide, recently shown to be a cytochrome P-450 of the IID subfamily, and (2) a new antigen of 66 kD, the location of which suggests it may also be part of the mono-oxygenase complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez F., Bernard O., Homberg J. C., Kreibich G. Anti-liver-kidney microsome antibody recognizes a 50,000 molecular weight protein of the endoplasmic reticulum. J Exp Med. 1985 May 1;161(5):1231–1236. doi: 10.1084/jem.161.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Gueguen M., Meunier-Rotival M., Bernard O., Alvarez F. Anti-liver kidney microsome antibody recognizes a cytochrome P450 from the IID subfamily. J Exp Med. 1988 Aug 1;168(2):801–806. doi: 10.1084/jem.168.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruppa J., Sabatini D. D. Release of poly A(+) messenger RNA from rat liver rough microsomes upon disassembly of bound polysomes. J Cell Biol. 1977 Aug;74(2):414–427. doi: 10.1083/jcb.74.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maggiore G., Bernard O., Homberg J. C., Hadchouel M., Alvarez F., Hadchouel P., Odièvre M., Alagille D. Liver disease associated with anti-liver-kidney microsome antibody in children. J Pediatr. 1986 Mar;108(3):399–404. doi: 10.1016/s0022-3476(86)80880-0. [DOI] [PubMed] [Google Scholar]
- Millward A., Hussain M. J., Peakman M., Pyke D. A., Leslie R. D., Vergani D. Characterization of islet cell antibody in insulin dependent diabetes: evidence for IgG1 subclass restriction and polyclonality. Clin Exp Immunol. 1988 Feb;71(2):353–356. [PMC free article] [PubMed] [Google Scholar]
- Odièvre M., Maggiore G., Homberg J. C., Saadoun F., Couroucé A. M., Yvart J., Hadchouel M., Alagille D. Seroimmunologic classification of chronic hepatitis in 57 children. Hepatology. 1983 May-Jun;3(3):407–409. doi: 10.1002/hep.1840030320. [DOI] [PubMed] [Google Scholar]
- Peakman M., Lobo-Yeo A., Mieli-Vergani G., Davies E. T., Mowat A. P., Vergani D. Characterization of anti-liver kidney microsomal antibody in childhood autoimmune chronic active hepatitis: evidence for IgG1 subclass restriction, polyclonality and non cross-reactivity with hepatocyte surface antigens. Clin Exp Immunol. 1987 Sep;69(3):543–549. [PMC free article] [PubMed] [Google Scholar]
- Rizzetto M., Swana G., Doniach D. Microsomal antibodies in active chronic hepatitis and other disorders. Clin Exp Immunol. 1973 Nov;15(3):331–344. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A. R., Bastin J., Gould K., Brownlee G. G. Cytotoxic T lymphocytes recognize influenza haemagglutinin that lacks a signal sequence. Nature. 1986 Dec 11;324(6097):575–577. doi: 10.1038/324575a0. [DOI] [PubMed] [Google Scholar]
- Vlasuk G. P., Walz F. G., Jr Liver endoplasmic reticulum polypeptides resolved by two-dimensional gel electrophoresis. Anal Biochem. 1980 Jun;105(1):112–120. doi: 10.1016/0003-2697(80)90431-5. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Lapenson D. P., Krishnan M., Bernard O., Kreibich G., Alvarez F. Antibodies to liver/kidney microsome1 in chronic active hepatitis recognize specific forms of hepatic cytochrome P-450. Gastroenterology. 1988 Nov;95(5):1326–1331. doi: 10.1016/0016-5085(88)90368-x. [DOI] [PubMed] [Google Scholar]
- Zouali M., Jefferis R., Eyquem A. IgG subclass distribution of autoantibodies to DNA and to nuclear ribonucleoproteins in autoimmune diseases. Immunology. 1984 Mar;51(3):595–600. [PMC free article] [PubMed] [Google Scholar]