Abstract
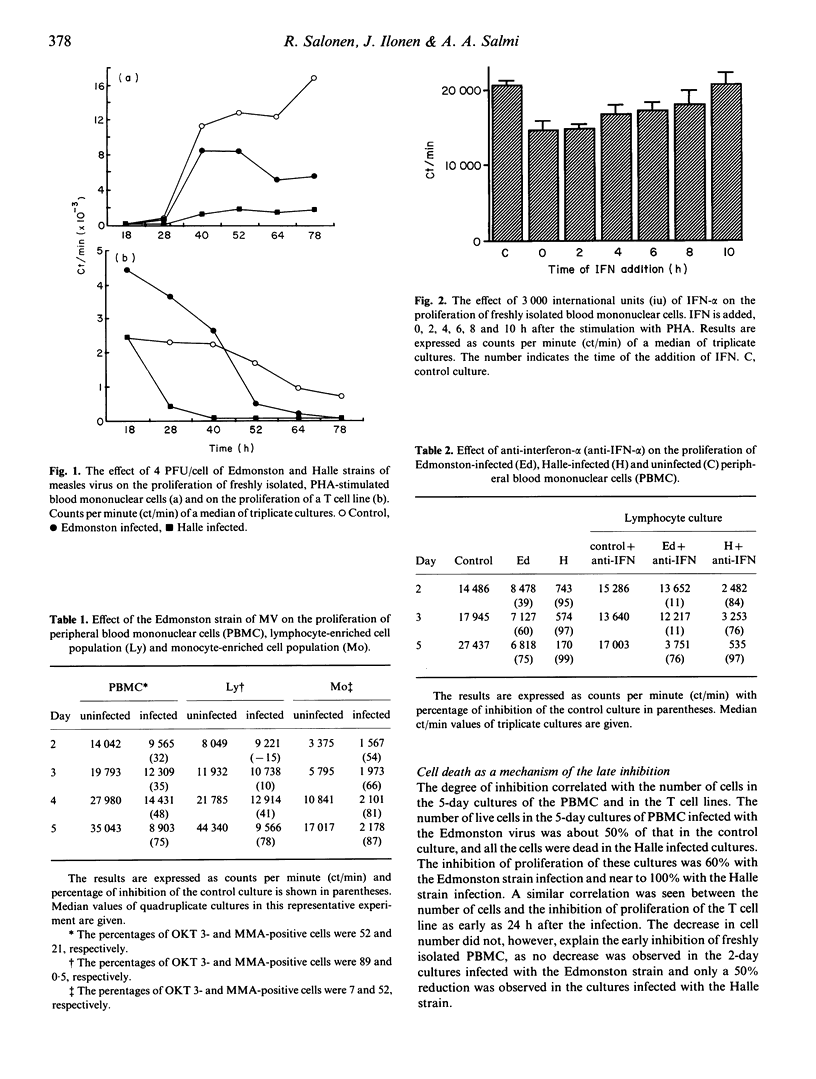
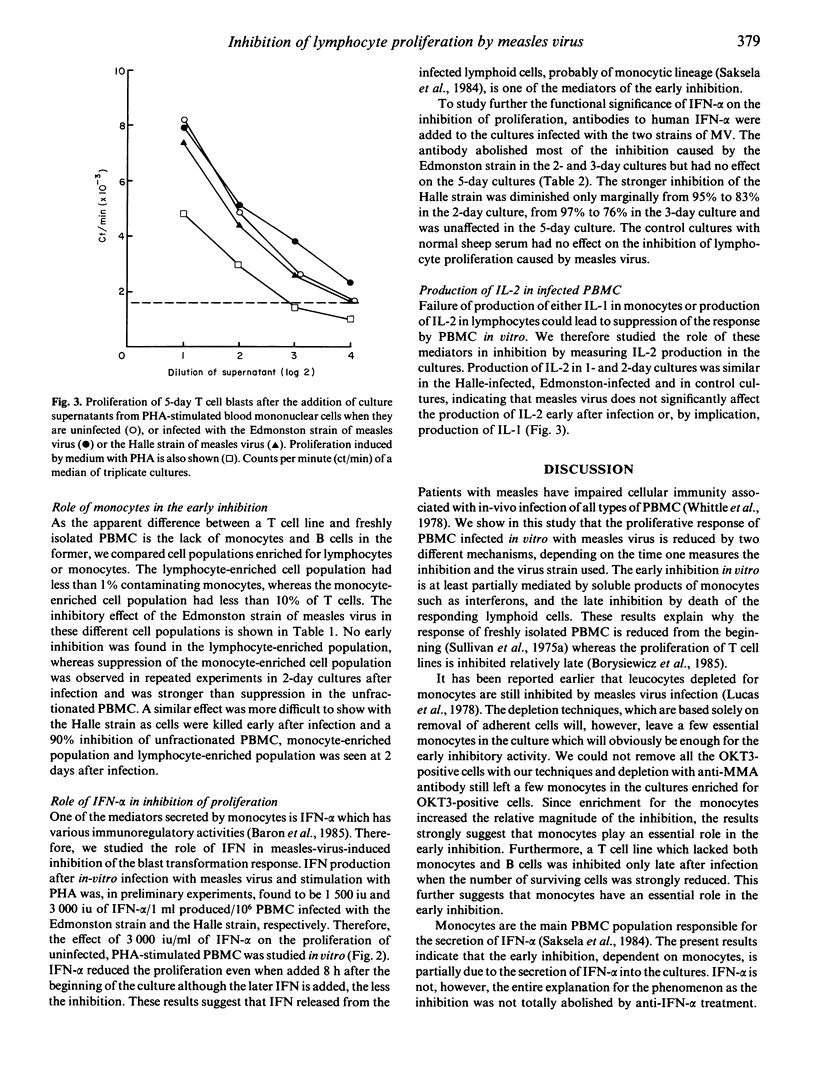
We studied the effect of two different strains of measles virus (MV) on the lymphocyte blast transformation response. Infectious virus inhibited the proliferation response to PHA early during the culture when freshly isolated PBMC were stimulated. However, MV stimulated the proliferation of T cell lines early after infection and the inhibition was a late phenomenon associated with cell death. The early inhibition by the Edmonston strain of MV was shown to be monocyte-dependent by depletion studies with monoclonal antibodies. It could be partially explained by IFN-alpha production as the addition of anti-IFN-alpha antiserum to the cultures reversed the virus-induced inhibition and the addition of IFN reduced the response of uninfected PBMC. The late inhibition by the Edmonston strain was associated neither with monocytes nor with IFN-alpha but correlated with cell death in cultures. The inhibition by the Halle strain of MV was strong and associated with cell death already early after infection. The results demonstrate that MV inhibits lymphocyte proliferation by two different mechanisms and different virus strains vary both in the magnitude and mechanism of inhibition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borysiewicz L. K., Casali P., Rogers B., Morris S., Sissons J. G. The immunosuppressive effects of measles virus on T cell function--failure to affect IL-2 release or cytotoxic T cell activity in vitro. Clin Exp Immunol. 1985 Jan;59(1):29–36. [PMC free article] [PubMed] [Google Scholar]
- Casali P., Rice G. P., Oldstone M. B. Viruses disrupt functions of human lymphocytes. Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J Exp Med. 1984 May 1;159(5):1322–1337. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galama J. M., Ubels-Postma J., Vos A., Lucas C. J. Measles virus inhibits acquisition of lymphocyte functions but not established effector functions. Cell Immunol. 1980 Mar 15;50(2):405–415. doi: 10.1016/0008-8749(80)90294-4. [DOI] [PubMed] [Google Scholar]
- Ilonen J., Salonen R., Marusyk R., Salmi A. Measles virus strain-dependent variation in outcome of infection of human blood mononuclear cells. J Gen Virol. 1988 Jan;69(Pt 1):247–252. doi: 10.1099/0022-1317-69-1-247. [DOI] [PubMed] [Google Scholar]
- Jacobson S., McFarland H. F. Measles virus persistence in human lymphocytes: a role for virus-induced interferon. J Gen Virol. 1982 Dec;63(2):351–357. doi: 10.1099/0022-1317-63-2-351. [DOI] [PubMed] [Google Scholar]
- Joffe M. I., Rabson A. R. Dissociation of lymphokine production and blastogenesis in children with measles infections. Clin Immunol Immunopathol. 1978 Jul;10(3):335–343. doi: 10.1016/0090-1229(78)90190-3. [DOI] [PubMed] [Google Scholar]
- Linnavuori K., Hovi T. Restricted replication of herpes simplex virus in human monocyte cultures: role of interferon. Virology. 1983 Oct 15;130(1):1–9. doi: 10.1016/0042-6822(83)90112-5. [DOI] [PubMed] [Google Scholar]
- Lucas C. J., Ubels-Postma J., Galama J. M., Rezee A. Studies on the mechanism of measles virus-induced suppression of lymphocyte functions in vitro: lack of a role for interferon and monocytes. Cell Immunol. 1978 May;37(2):448–458. doi: 10.1016/0008-8749(78)90212-5. [DOI] [PubMed] [Google Scholar]
- McChesney M. B., Fujinami R. S., Lampert P. W., Oldstone M. B. Viruses disrupt functions of human lymphocytes. II. Measles virus suppresses antibody production by acting on B lymphocytes. J Exp Med. 1986 May 1;163(5):1331–1336. [PMC free article] [PubMed] [Google Scholar]
- Neighbour P. A., Bloom B. R. Absence of virus-induced lymphocyte suppression and interferon production in multiple sclerosis. Proc Natl Acad Sci U S A. 1979 Jan;76(1):476–480. doi: 10.1073/pnas.76.1.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osunkoya B. O., Adeleye G. I., Adejumo T. A., Salimonu L. S. Studies on leukocyte cultures in measles. II. Detection of measles virus antigen in human leucocytes by immunofluorescence. Arch Gesamte Virusforsch. 1974;44(4):323–329. doi: 10.1007/BF01251013. [DOI] [PubMed] [Google Scholar]
- Roberts N. J., Jr Different effects of influenza virus, respiratory syncytial virus, and Sendai virus on human lymphocytes and macrophages. Infect Immun. 1982 Mar;35(3):1142–1146. doi: 10.1128/iai.35.3.1142-1146.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen R., Ilonen J., Salmi A. Measles virus infection of unstimulated blood mononuclear cells in vitro: antigen expression and virus production preferentially in monocytes. Clin Exp Immunol. 1988 Feb;71(2):224–228. [PMC free article] [PubMed] [Google Scholar]
- Soontiëns F. J., van der Veen J. Evidence for a macrophage-mediated effect of poliovirus on the lymphocyte response to phytohemagglutinin. J Immunol. 1973 Nov;111(5):1411–1419. [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Albrecht P., Lucas S. J. Inhibition of lymphocyte stimulation by measles virus. J Immunol. 1975 May;114(5):1458–1461. [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Lucas S. J., Albrecht P. Measles infection of human mononuclear cells. I. Acute infection of peripheral blood lymphocytes and monocytes. J Exp Med. 1975 Sep 1;142(3):773–784. doi: 10.1084/jem.142.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg M. A., Roy S., Vydelingum S., Grise-Miron L. The role of interleukins in viral inhibition of lymphocyte mitogenesis. J Clin Lab Immunol. 1985 Dec;18(4):167–174. [PubMed] [Google Scholar]
- Whittle H. C., Dossetor J., Oduloju A., Bryceson A. D., Greenwood B. M. Cell-mediated immunity during natural measles infection. J Clin Invest. 1978 Sep;62(3):678–684. doi: 10.1172/JCI109175. [DOI] [PMC free article] [PubMed] [Google Scholar]


