Figure 4.
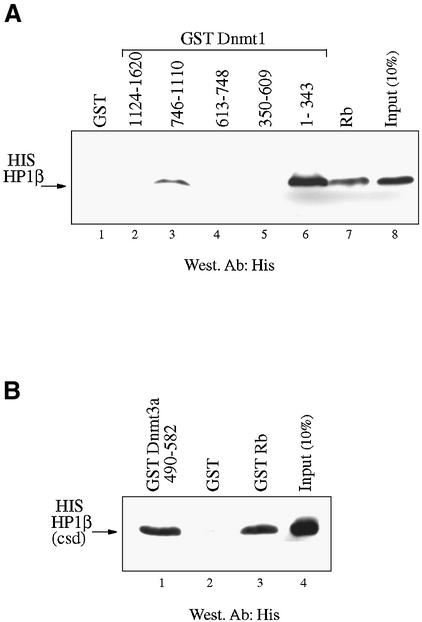
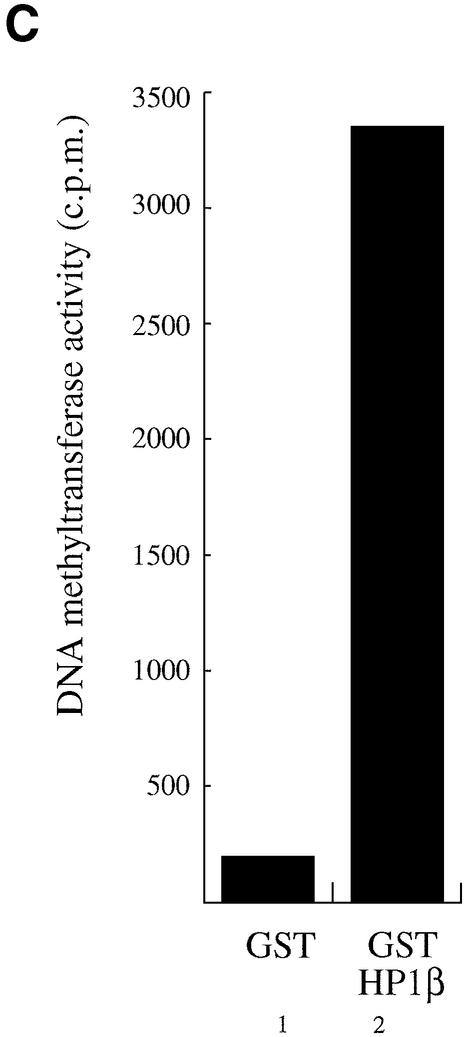
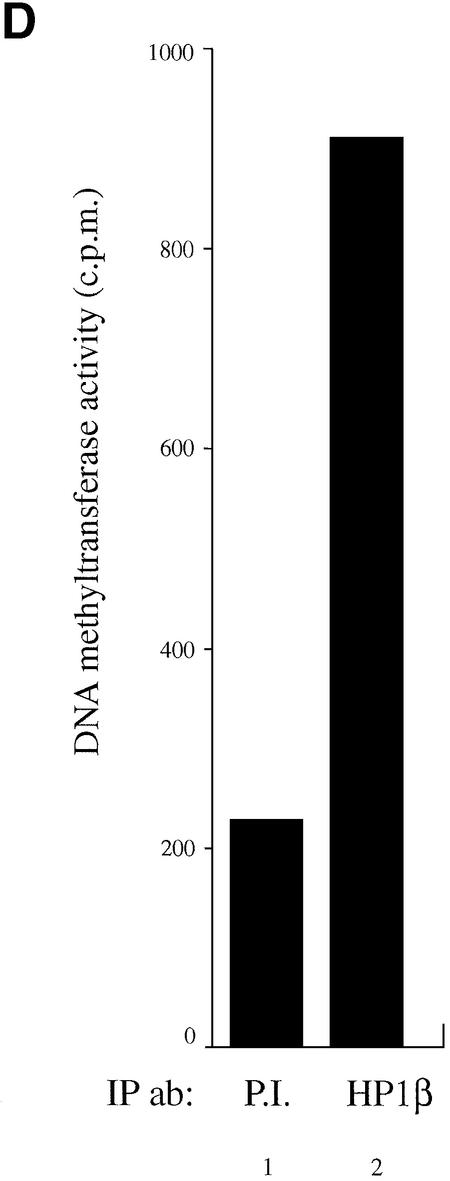
HP1β binds directly to Dnmt1 and Dnmt3a and interacts in vitro and in vivo with DNA methyltransferase activity. (A) HP1β directly contacts Dnmt1. GST, GST–Rb or the indicated GST–Dnmt1 fusions were incubated with histidine-tagged full-length HP1β. Direct binding was visualised by western blotting for HP1β using anti-His antibody (Roche). Input is 10% of total His-tagged HP1β in each assay. (B) HP1β binds directly to Dnmt3a. GST, GST–Rb or GST–Dnmt3a containing its PHD-like motif were incubated with histidine-tagged HP1β chromo-shadow domain (His-HP1β csd, residues 104–185). Western blotting was then performed using anti-His antibody. Input is 10% of total His-tagged HP1β chromo-shadow domain in each assay. (C) HP1β binds in vitro with DNA methyltransferase activity. Equivalent amounts of GST (lane 1) or GST–HP1β fusion proteins (lane 2) were incubated with HeLa nuclear extract. After extensive washing, the beads were assayed for DNA methyltransferase activity. Activity is given as c.p.m. of S-adenosyl-l-[methyl-3H]methionine incorporated into a hemi-methylated oligonucleotide substrate. Incorporation of radioactivity was determined by liquid scintillation counting. (D) Endogenous HP1β purifies DNA methyltransferase activity from nuclear extracts. HeLa nuclear extracts were immunoprecipitated with preimmune serum (lane 1) or an anti-HP1β antibody (lane 2). Immunoprecipitates were then assayed for DNA methyltransferase activity.