Abstract
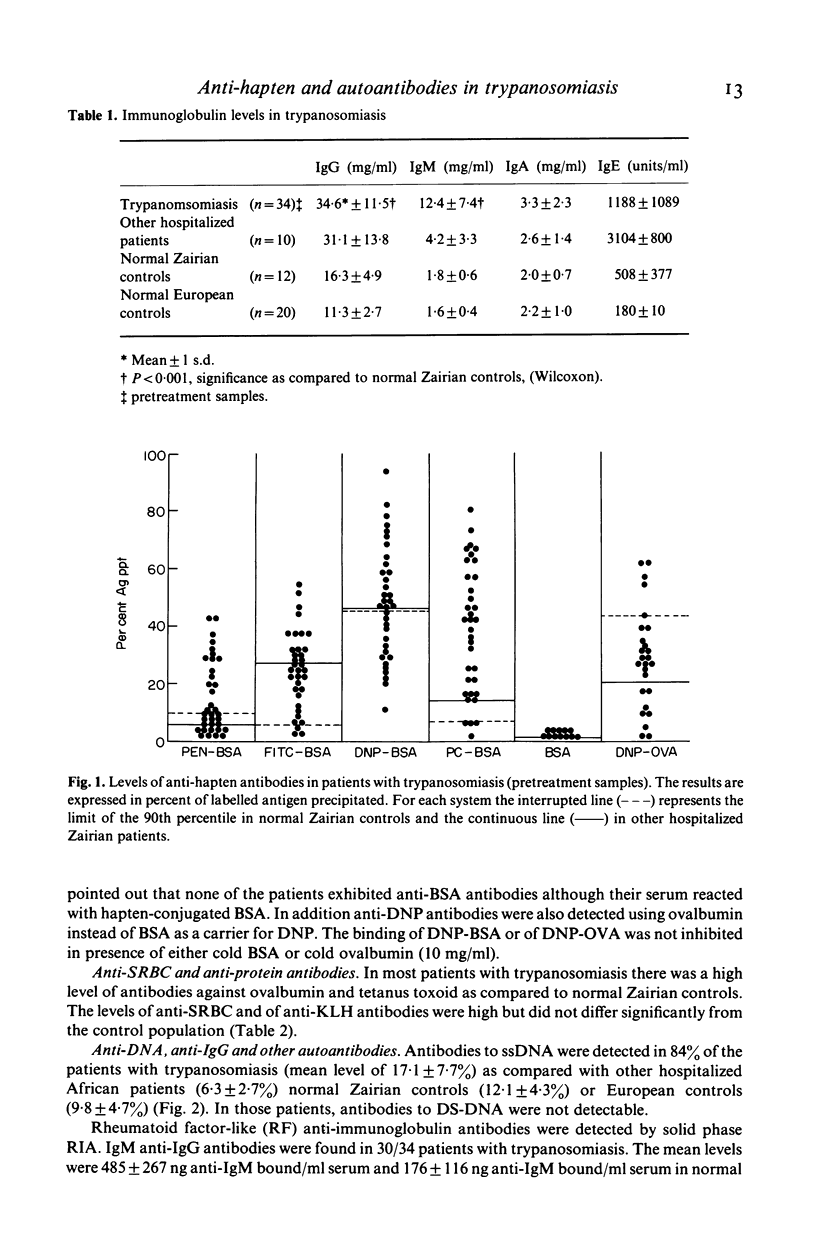
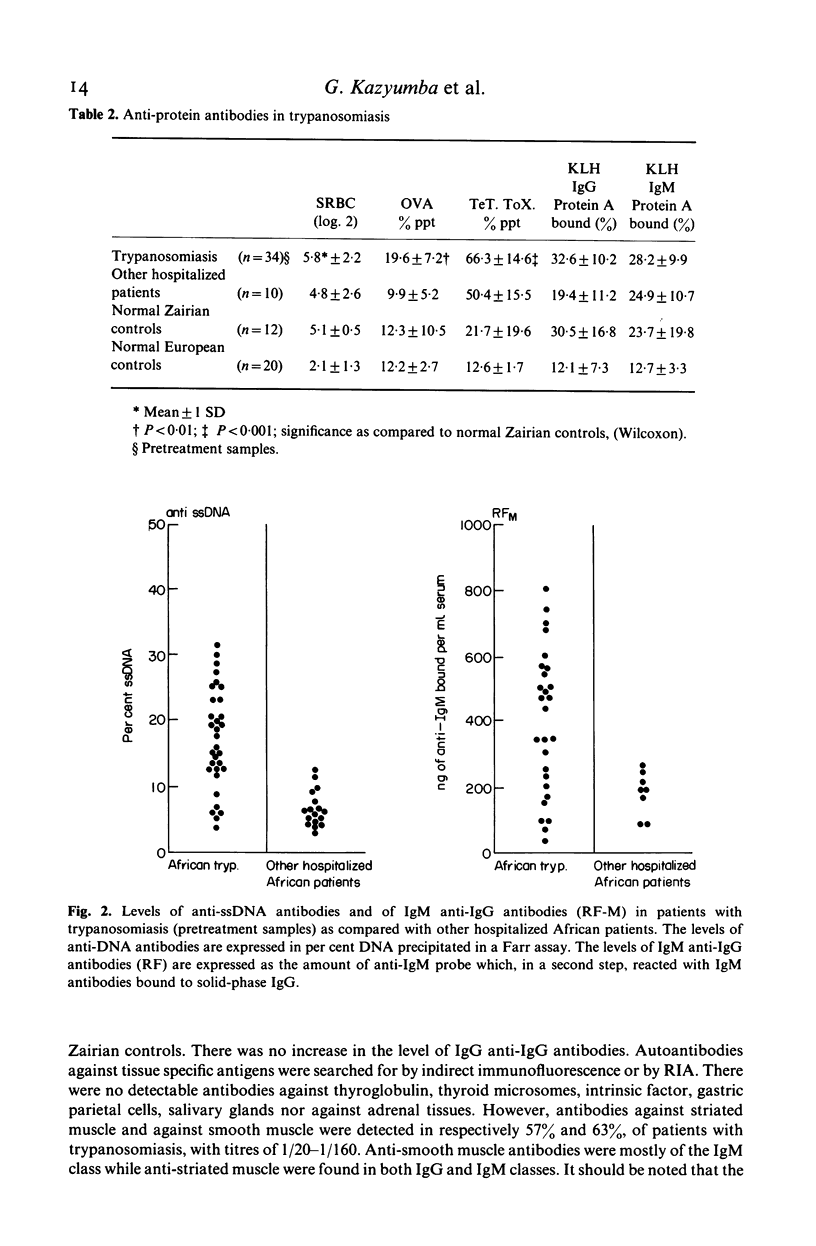
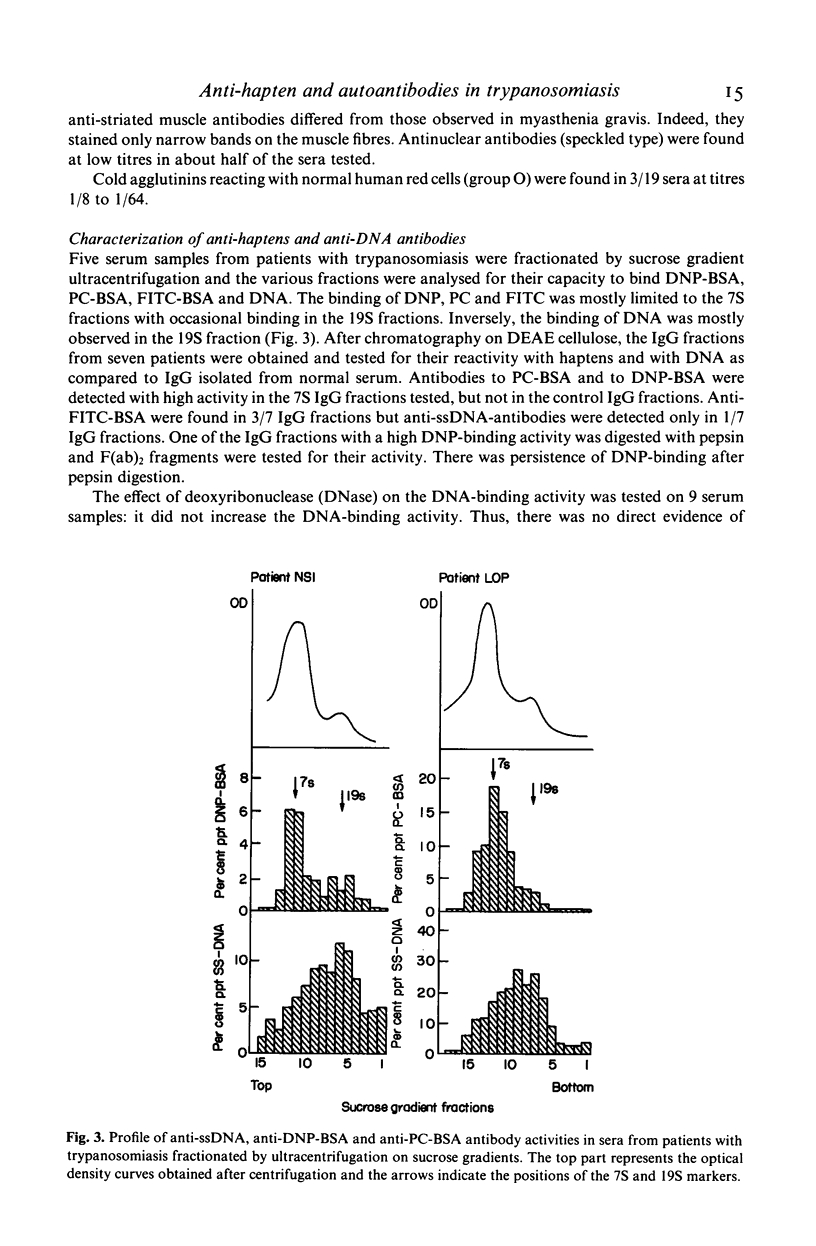
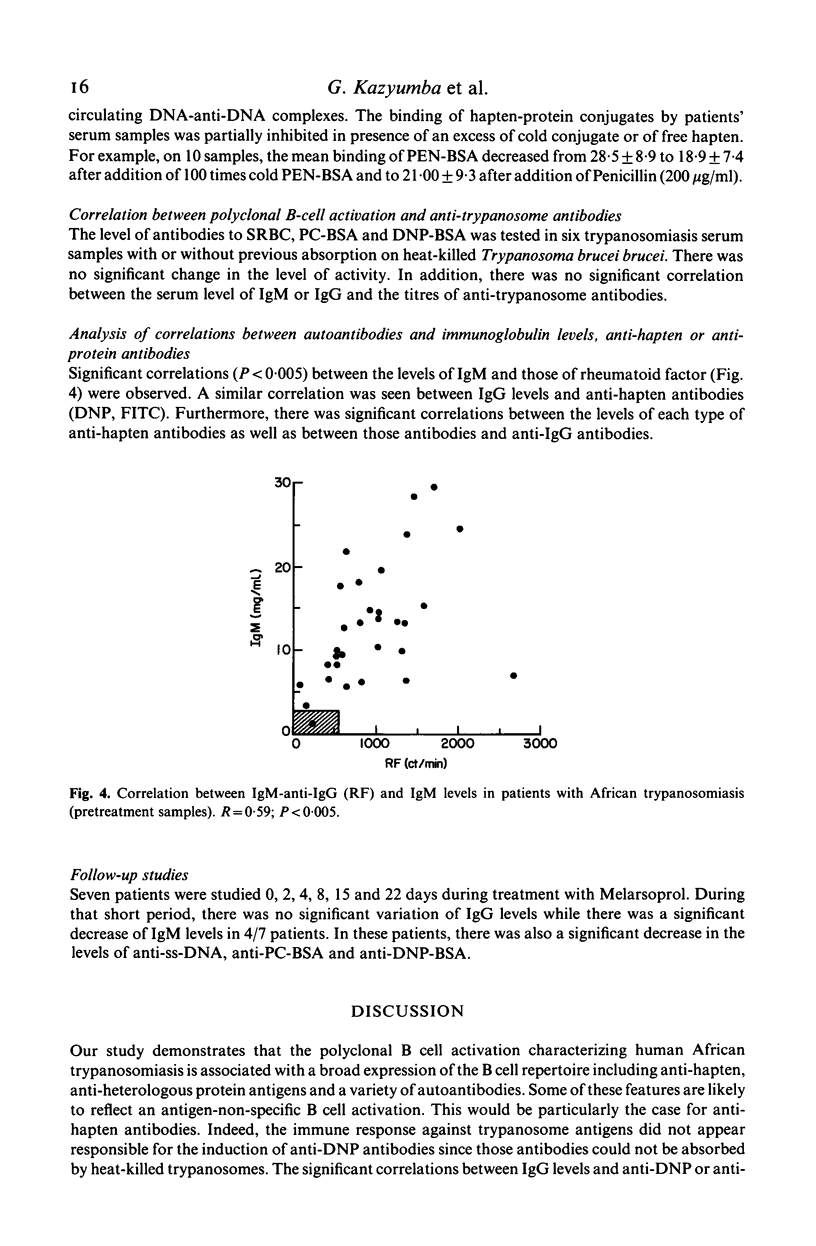
The relative importance of polyclonal B cell activation has been studied in relation to the development of autoantibodies in human African trypanosomiasis. In 34 patients investigated before specific treatment a broad expression of the B cell repertoire was observed including the production of anti-hapten (FITC, Penicillin, Phosphorylcholin) antibodies, of high levels of antibodies against some heterologous protein antigens (ovalbumin and tetanus toxoid) and of autoantibodies. Anti-ssDNA antibodies were detected in 84% of the patients and anti-IgG rheumatoid factors in 88%. Anti-striated muscle and anti-smooth muscle antibodies were also observed in 57 and 63% of the patients. Correlation analysis indicated that the formation of anti-DNA antibodies is associated with polyclonal B cell activation but probably depends on an additional B cell stimulation by released DNA or cross-reacting antigens. Anti-immunoglobulin antibodies are closely correlated with polyclonal B cell activation and their production is likely to reflect the high frequency of anti-IgG B cell precursors in the normal human B cell repertoire. The significance of these observations in relation to the pathological expression of trypanosomiasis should be particularly considered in the generation of immune complexes either in circulating blood or locally at the sites of parasite destruction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carroll P., Stafford D., Schwartz R. S., Stollar B. D. Murine monoclonal anti-DNA autoantibodies bind to endogenous bacteria. J Immunol. 1985 Aug;135(2):1086–1090. [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Freeman T., Smithers S. R., Targett G. A., Walk P. J. Specificity of immunoglobulin G in rhesus monkeys infected with Schistosoma mansoni, Plasmodium knowlesi, and Trypanosoma brucei. J Infect Dis. 1970 Apr;121(4):401–406. doi: 10.1093/infdis/121.4.401. [DOI] [PubMed] [Google Scholar]
- Galvão-Castro B., Sá Ferreira J. A., Marzochi K. F., Marzochi M. C., Coutinho S. G., Lambert P. H. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human american visceral leishmaniasis. Clin Exp Immunol. 1984 Apr;56(1):58–66. [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M. Possible role of a B-cell mitogen in hypergammaglobulinaemia in malaria and trypanosomiasis. Lancet. 1974 Mar 16;1(7855):435–436. doi: 10.1016/s0140-6736(74)92386-1. [DOI] [PubMed] [Google Scholar]
- Houba V., Brown K. N., Allison A. C. Heterophile antibodies, M-antiglobulins and immunoglobulins in experimental trypanosomiasis. Clin Exp Immunol. 1969 Jan;4(1):113–123. [PMC free article] [PubMed] [Google Scholar]
- Hudson K. M., Byner C., Freeman J., Terry R. J. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature. 1976 Nov 18;264(5583):256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. IgM rheumatoid factors in mice injected with bacterial lipopolysaccharides. J Immunol. 1979 May;122(5):2096–2102. [PubMed] [Google Scholar]
- Izui S., Lambert P. H., Miescher P. A. Determination of anti-DNA antibodies by a modified 125I-labelled DNA-binding test. Elimination of non-specific binding of DNA to non-immunoglobulin basic proteins by using an anionic detergent. Clin Exp Immunol. 1976 Dec;26(3):425–430. [PMC free article] [PubMed] [Google Scholar]
- Lambert P. H., Berney M., Kazyumba G. Immune complexes in serum and in cerebrospinal fluid in African trypanosomiasis. Correlation with polyclonal B cell activation and with intracerebral immunoglobulin synthesis. J Clin Invest. 1981 Jan;67(1):77–85. doi: 10.1172/JCI110035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Musoke A. J., Nantulya V. M., Barbet A. F., Kironde F., McGuire T. C. Bovine immune response to African ;trypanosomes: specific antibodies to variable surface glycoproteins of Trypanosoma brucei. Parasite Immunol. 1981 Summer;3(2):97–106. doi: 10.1111/j.1365-3024.1981.tb00388.x. [DOI] [PubMed] [Google Scholar]
- Novogrodsky A., Rubin A. L., Stenzel K. H. Selective suppression by adherent cells, prostaglandin, and cyclic AMP analogues of blastogenesis induced by different mitogens. J Immunol. 1979 Jan;122(1):1–7. [PubMed] [Google Scholar]
- Rose L. M., Goldman M., Lambert P. H. Simultaneous induction of an idiotype, corresponding anti-idiotypic antibodies, and immune complexes during African trypanosomiasis in mice. J Immunol. 1982 Jan;128(1):79–85. [PubMed] [Google Scholar]


