Abstract
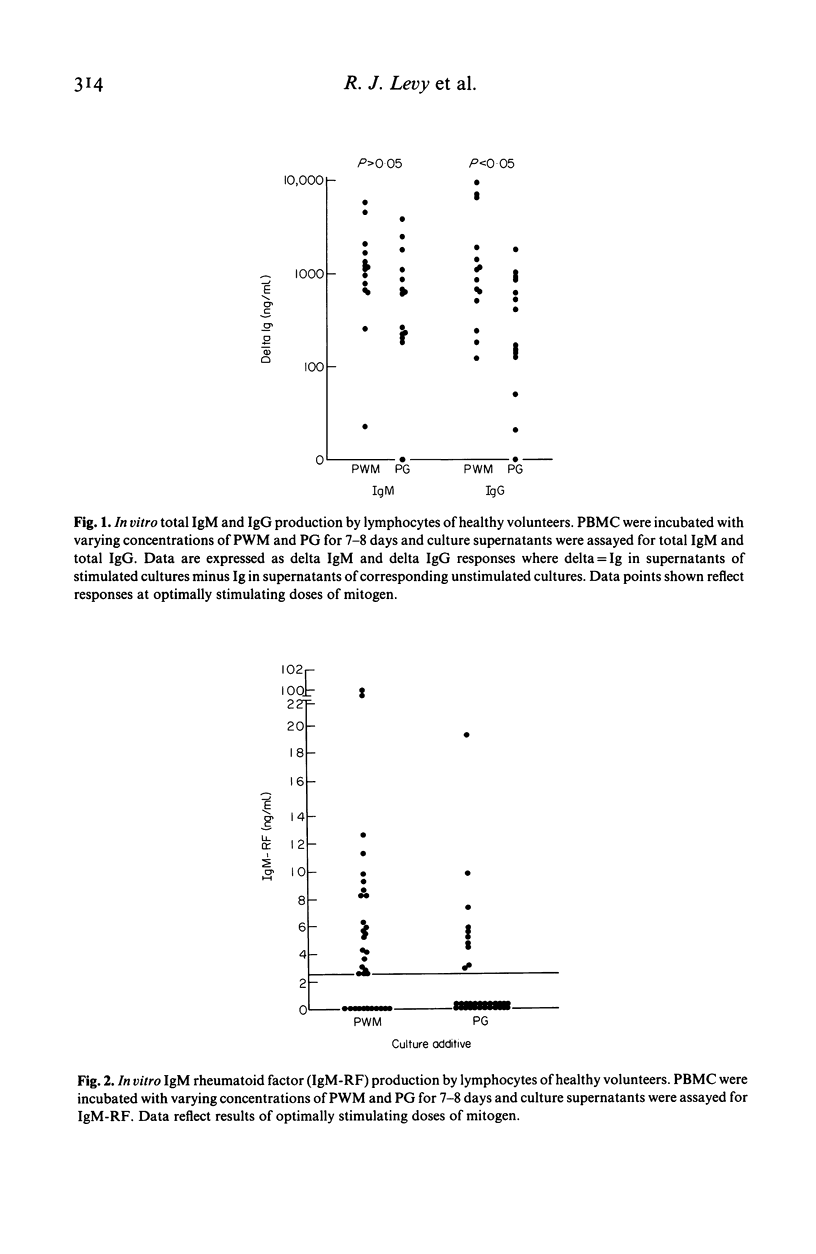
The present studies were carried out to further characterize the polyclonal B cell activating properties of bacterial peptidoglycan (PG) and to determine if this ubiquitous agent induces in vitro IgM rheumatoid factor (RF) production by lymphocytes from healthy volunteers. Peripheral blood mononuclear cells (PBMC) were cultured in the presence of peptidoglycan, pokeweed mitogen (PWM), a standard polyclonal B cell activator, or additional culture medium. Supernatants were harvested on days 7-8 for determination of total IgM, total IgG, and IgM RF by an enzyme-linked immunosorbent assay (ELISA). PG and PWM induced comparable amounts of total IgM production but PG was a less potent stimulant of total IgG production. PG induced in vitro IgM-RF production in 9/33 experiments, a frequency of response of less than that observed in corresponding PWM stimulated cultures (22/33 experiments). PG-induced IgM-RF production depended upon active protein synthesis and did not correlate with the magnitude of PG-induced total IgM production. The latter finding suggests that PG-induced IgM-RF may not merely reflect polyclonal B cell activation. These results add to a growing list of PG's functional properties and provide further impetus for considering this ubiquitous agent as a potential stimulant for in vivo RF production.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Anti-immunoglobulin autoantibodies are not preferentially induced in polyclonal activation of human and mouse lymphocytes, and more anti-DNA and anti-erythrocyte autoantibodies are induced in polyclonal activation of mouse than human lymphocytes. J Immunol. 1984 Nov;133(5):2537–2544. [PubMed] [Google Scholar]
- Dziarski R., Dziarski A., Levinson A. I. Mitogenic responsiveness of mouse, rat and human lymphocytes to Staphylococcus aureus cell wall, teichoic acid, and peptidoglycan. Int Arch Allergy Appl Immunol. 1980;63(4):383–395. doi: 10.1159/000232654. [DOI] [PubMed] [Google Scholar]
- Dziarski R., Dziarski A. Mitogenic activity of staphylococcal peptidoglycan. Infect Immun. 1979 Mar;23(3):706–710. doi: 10.1128/iai.23.3.706-710.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Opposing effects of xid and nu mutations on proliferative and polyclonal antibody and autoantibody responses to peptidoglycan, LPS, protein A and PWM. Immunology. 1984 Nov;53(3):563–574. [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Polyclonal activation of immunoglobulin secretion in B lymphocytes induced by staphylococcal peptidoglycan. J Immunol. 1980 Dec;125(6):2478–2483. [PubMed] [Google Scholar]
- Fong S., Vaughan J. H., Carson D. A. Two different rheumatoid factor-producing cell populations distinguished by the mouse erythrocyte receptor and responsiveness to polyclonal B cell activators. J Immunol. 1983 Jan;130(1):162–164. [PubMed] [Google Scholar]
- Hang L., Slack J. H., Amundson C., Izui S., Theofilopoulos A. N., Dixon F. J. Induction of murine autoimmune disease by chronic polyclonal B cell activation. J Exp Med. 1983 Mar 1;157(3):874–883. doi: 10.1084/jem.157.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. IgM rheumatoid factors in mice injected with bacterial lipopolysaccharides. J Immunol. 1979 May;122(5):2096–2102. [PubMed] [Google Scholar]
- Koopman W. J., Schrohenloher R. E. In vitro synthesis of IgM rheumatoid factor by lymphocytes from healthy adults. J Immunol. 1980 Aug;125(2):934–939. [PubMed] [Google Scholar]
- Levinson A. I., Dziarski A., Zweiman B., Dziarski R. Staphylococcal peptidoglycan: T-cell-dependent mitogen and relatively T-cell-independent polyclonal B-cell activator of human lymphocytes. Infect Immun. 1983 Jan;39(1):290–296. doi: 10.1128/iai.39.1.290-296.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo I., Carafa C., Dziarski R., Levinson A. I. Analysis of in vitro polyclonal B cell differentiation responses to bacterial peptidoglycan and pokeweed mitogen in rheumatoid arthritis. Clin Exp Immunol. 1984 May;56(2):253–262. [PMC free article] [PubMed] [Google Scholar]
- Pardo I., Levinson A. I. Circulating immunoglobulin-secreting cells in rheumatoid arthritis. Clin Immunol Immunopathol. 1983 Oct;29(1):29–34. doi: 10.1016/0090-1229(83)90004-1. [DOI] [PubMed] [Google Scholar]
- Park H. A simple washing technique for solid-phase radioimmunoassays and enzyme-linked immunosorbent assays. J Clin Pathol. 1981 Nov;34(11):1309–1310. doi: 10.1136/jcp.34.11.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali J. L., Fong S., Tsoukas C. D., Slovin S. F., Vaughan J. H., Carson D. A. Different populations of rheumatoid factor idiotypes induced by two polyclonal B cell activators, pokeweed mitogen and Epstein--Barr virus. Clin Immunol Immunopathol. 1981 Nov;21(2):184–189. doi: 10.1016/0090-1229(81)90207-5. [DOI] [PubMed] [Google Scholar]
- Primi D., Hammarström L., Smith C. I., Möller G. Characterization of self-reactive B cells by polyclonal B-cell activators. J Exp Med. 1977 Jan 1;145(1):21–30. doi: 10.1084/jem.145.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter L., Carson D. A., Jensen F. C., Holbrook T. L., Vaughan J. H. In vitro effects of Epstein-Barr virus on peripheral blood mononuclear cells from patients with rheumatoid arthritis and normal subjects. J Exp Med. 1978 Nov 1;148(5):1429–1434. doi: 10.1084/jem.148.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]


