Abstract
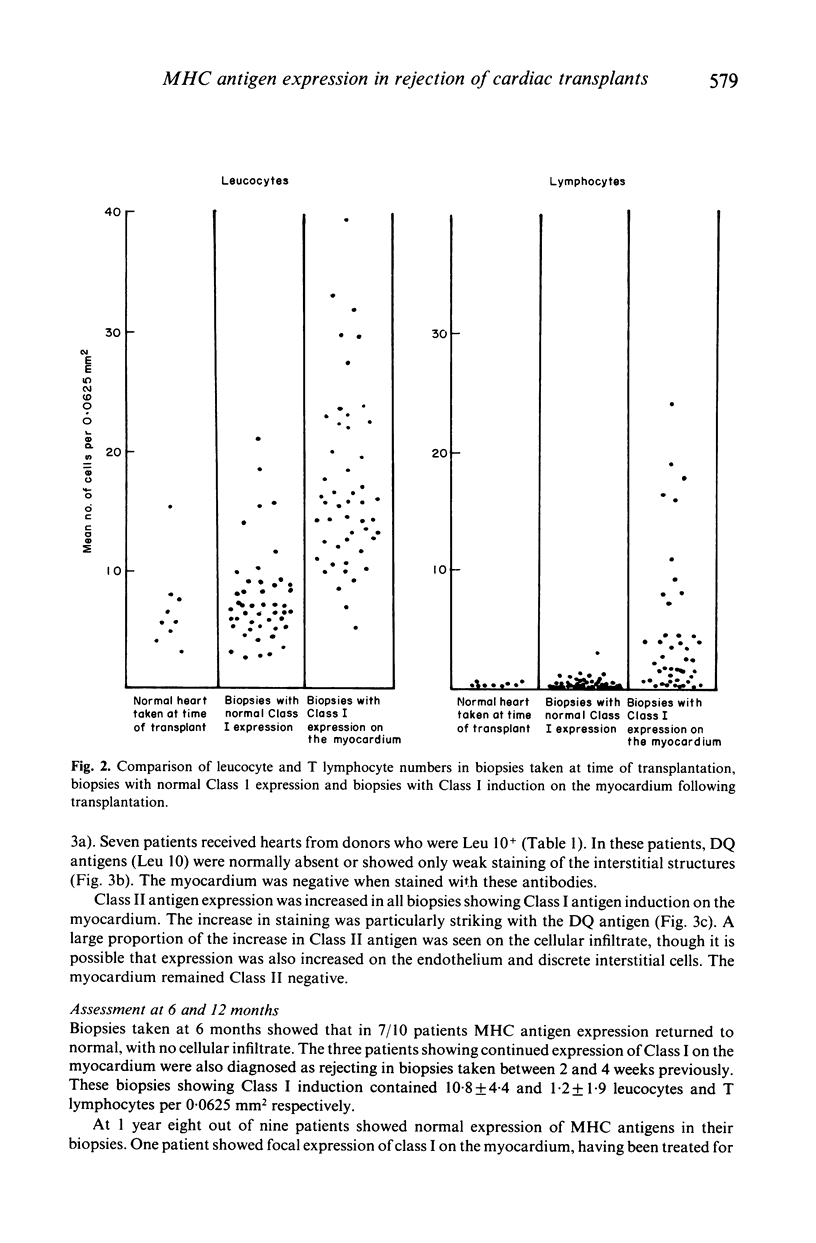
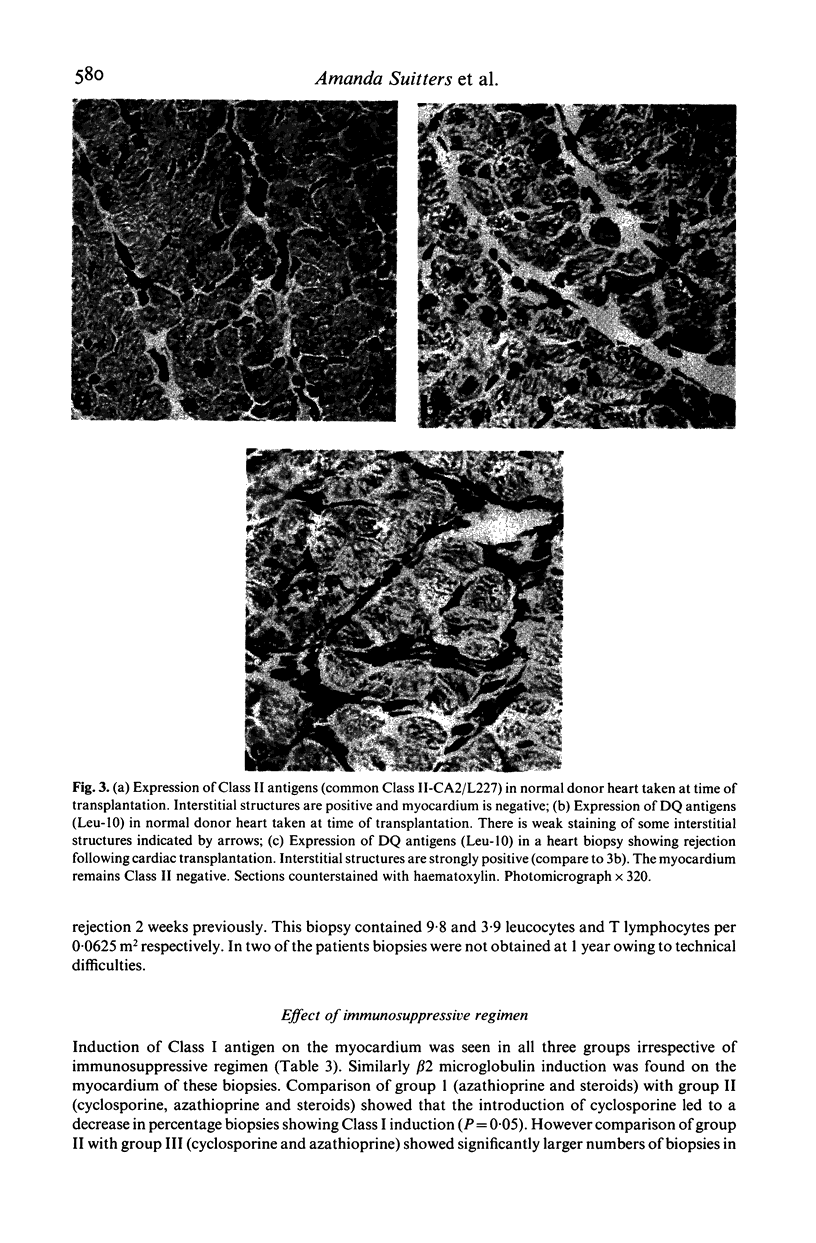
Class I induction on the myocardium of transplanted heart was investigated with regard to its temporal relationship to rejection episodes, how it is affected by anti-rejection therapy and whether it is dependent upon the presence of a T cell infiltrate in the biopsy. Sequential cardiac biopsies (total 114) from 11 patients from the time of transplant to 1 year after transplant were studied using immunocytochemical techniques. The effect of different immunosuppressive regimens on MHC antigen expression was also studied. All the biopsies diagnosed as showing rejection for the first time showed induction of Class 1 on the myocardium with 79% during subsequent rejection episodes. Class I induction was associated with a leucocyte infiltrate, not always containing T cells, and disappeared in 47% of biopsies taken 3-4 weeks after treatment with steroids and/or ATG. Increased expression of Class II, in particular DQ antigens on interstitial structures, paralleled Class 1 induction. MHC antigen expression returned to normal in 8/9 patients, at 1 year after transplant. Different immunosuppressive regimens affected the number of biopsies showing Class 1 induction on the myocardium. Our results suggest that in clinical heart transplantation class I induction is related to the rejection process.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autenried P., Halloran P. F. Cyclosporine blocks the induction of class I and class II MHC products in mouse kidney by graft-vs-host disease. J Immunol. 1985 Dec;135(6):3922–3928. [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Benacerraf B., Germain R. N. The immune response genes of the major histocompatibility complex. Immunol Rev. 1978;38:70–119. doi: 10.1111/j.1600-065x.1978.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Berah M., Hors J., Dausset J. A study of HL-A antigens in human organs. Transplantation. 1970 Mar;9(3):185–192. doi: 10.1097/00007890-197003000-00001. [DOI] [PubMed] [Google Scholar]
- Blackman M. J., Morris A. G. The effect of interferon treatment of targets on susceptibility to cytotoxic T-lymphocyte killing: augmentation of allogeneic killing and virus-specific killing relative to viral antigen expression. Immunology. 1985 Nov;56(3):451–457. [PMC free article] [PubMed] [Google Scholar]
- Capobianchi M. R., Ameglio F., Tosi R., Dolei A. Differences in the expression and release of DR, BR, and DQ molecules in human cells treated with recombinant interferon gamma: comparison to other interferons. Hum Immunol. 1985 May;13(1):1–11. doi: 10.1016/0198-8859(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984 Sep;38(3):287–292. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur J Immunol. 1980 Oct;10(10):737–744. doi: 10.1002/eji.1830101003. [DOI] [PubMed] [Google Scholar]
- Fellous M., Kamoun M., Gresser I., Bono R. Enhanced expression of HLA antigens and beta 2-microglobulin on interferon-treated human lymphoid cells. Eur J Immunol. 1979 Jun;9(6):446–449. doi: 10.1002/eji.1830090606. [DOI] [PubMed] [Google Scholar]
- Fuggle S. V., Errasti P., Daar A. S., Fabre J. W., Ting A., Morris P. J. Localization of major histocompatibility complex (HLA-ABC and DR) antigens in 46 kidneys. Differences in HLA-DR staining of tubules among kidneys. Transplantation. 1983 Apr;35(4):385–390. doi: 10.1097/00007890-198304000-00024. [DOI] [PubMed] [Google Scholar]
- Fuggle S. V., McWhinnie D. L., Chapman J. R., Taylor H. M., Morris P. J. Sequential analysis of HLA-class II antigen expression in human renal allografts. Induction of tubular class II antigens and correlation with clinical parameters. Transplantation. 1986 Aug;42(2):144–150. doi: 10.1097/00007890-198608000-00008. [DOI] [PubMed] [Google Scholar]
- Gibbs V. C., Wood D. M., Garovoy M. R. The response of cultured human kidney capillary endothelium to immunologic stimuli. Hum Immunol. 1985 Nov;14(3):259–269. doi: 10.1016/0198-8859(85)90233-2. [DOI] [PubMed] [Google Scholar]
- Green N., Hiai H., Elder J. H., Schwartz R. S., Khiroya R. H., Thomas C. Y., Tsichlis P. N., Coffin J. M. Expression of leukemogenic recombinant viruses associated with a recessive gene in HRS/J mice. J Exp Med. 1980 Aug 1;152(2):249–264. doi: 10.1084/jem.152.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen G., Buurman W. A., van der Linden C. J. Lymphokine dependence of in vivo expression of MHC class II antigens by endothelium. Nature. 1985 Jul 25;316(6026):361–363. doi: 10.1038/316361a0. [DOI] [PubMed] [Google Scholar]
- Groenewegen G., de Ley M., Jeunhomme G. M., Buurman W. A. Supernatants of human leukocytes contain mediator, different from interferon gamma, which induces expression of MHC class II antigens. J Exp Med. 1986 Jul 1;164(1):131–143. doi: 10.1084/jem.164.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg H., Moen T., Throsby E. Specific destruction of human endothelial cell monolayers by anti-DRw antisera. Transplantation. 1979 Aug;28(2):116–120. doi: 10.1097/00007890-197908000-00009. [DOI] [PubMed] [Google Scholar]
- Lampert I. A., Janossy G., Suitters A. J., Bofill M., Palmer S., Gordon-Smith E., Prentice H. G., Thomas J. A. Immunological analysis of the skin in graft versus host disease. Clin Exp Immunol. 1982 Oct;50(1):123–131. [PMC free article] [PubMed] [Google Scholar]
- Milton A. D., Fabre J. W. Massive induction of donor-type class I and class II major histocompatibility complex antigens in rejecting cardiac allografts in the rat. J Exp Med. 1985 Jan 1;161(1):98–112. doi: 10.1084/jem.161.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali P. G., De Martino C., Quaranta V., Nicotra M. R., Frezza F., Pellegrino M. A., Ferrone S. Expression of Ia-like antigens in normal human nonlymphoid tissues. Transplantation. 1981 Jan;31(1):75–78. doi: 10.1097/00007890-198101000-00017. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Collins T., Gimbrone M. A., Jr, Libby P., Reiss C. S. Inducible expression of class II major histocompatibility complex antigens and the immunogenicity of vascular endothelium. Transplantation. 1986 Feb;41(2):141–146. doi: 10.1097/00007890-198602000-00001. [DOI] [PubMed] [Google Scholar]
- Reem G. H., Cook L. A., Vilcek J. Gamma interferon synthesis by human thymocytes and T lymphocytes inhibited by cyclosporin A. Science. 1983 Jul 1;221(4605):63–65. doi: 10.1126/science.6407112. [DOI] [PubMed] [Google Scholar]
- Rose M. L., Coles M. I., Griffin R. J., Pomerance A., Yacoub M. H. Expression of class I and class II major histocompatibility antigens in normal and transplanted human heart. Transplantation. 1986 Jun;41(6):776–780. doi: 10.1097/00007890-198606000-00021. [DOI] [PubMed] [Google Scholar]
- Wagner C. R., Vetto R. M., Burger D. R. Subcultured human endothelial cells can function independently as fully competent antigen-presenting cells. Hum Immunol. 1985 May;13(1):33–47. doi: 10.1016/0198-8859(85)90025-4. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]




