Figure 1.
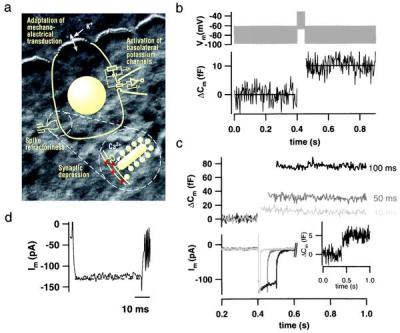
Exocytosis recorded by Cm measurements from single IHCs. (a) Simplified representation of an IHC, the measuring configuration, and an afferent IHC synapse (Inset) overlaid onto a Nomarski image of the explanted mouse organ of Corti (stereocilia of the row of IHCs are shown at the top of the image; cell bodies are covered by supporting cells). Previously suggested mechanisms of adaptation are indicated according to their localizations. Synaptic depression could be due to Ca2+ current inactivation, vesicle depletion, or postsynaptic glutamate receptor desensitization. (b) IHCs kept at 35°C in 2 mM [Ca2+]e were stimulated by a 50-ms depolarizing sinusoidal voltage (1 kHz; 40 mV peak to peak added to a dc potential of −50 mV; Upper). Four Cm traces recorded from three cells in the whole-cell configuration (0.1 mM EGTA added to the pipette solution) were averaged and smoothed by box averaging (n = 3; Lower; horizontal lines represent Cm averages before and after stimulation). Cm estimation is not valid during the depolarization because of nonlinear conductance changes. (c) High time resolution plot of ΔCm and membrane currents measured in response to depolarizations (to −15 mV) of different durations in the perforated-patch configuration. ΔCm traces were smoothed by box averaging (n = 3), and current traces were smoothed by binomial smoothing (n = 5). Outward currents were (incompletely) inhibited by intracellular Cs+ and extracellular tetraethylammonium (35 mM). (Inset) Average of four ΔCm traces in response to 4-ms depolarizations (to −15 mV). (d) Representative membrane current response to a 50-ms depolarization to −15 mV in the presence of intracellular (13 mM) and extracellular (35 mM) tetraethylammonium. Very little decline (inactivation) of Ca2+ current was observed under these conditions.
