Abstract
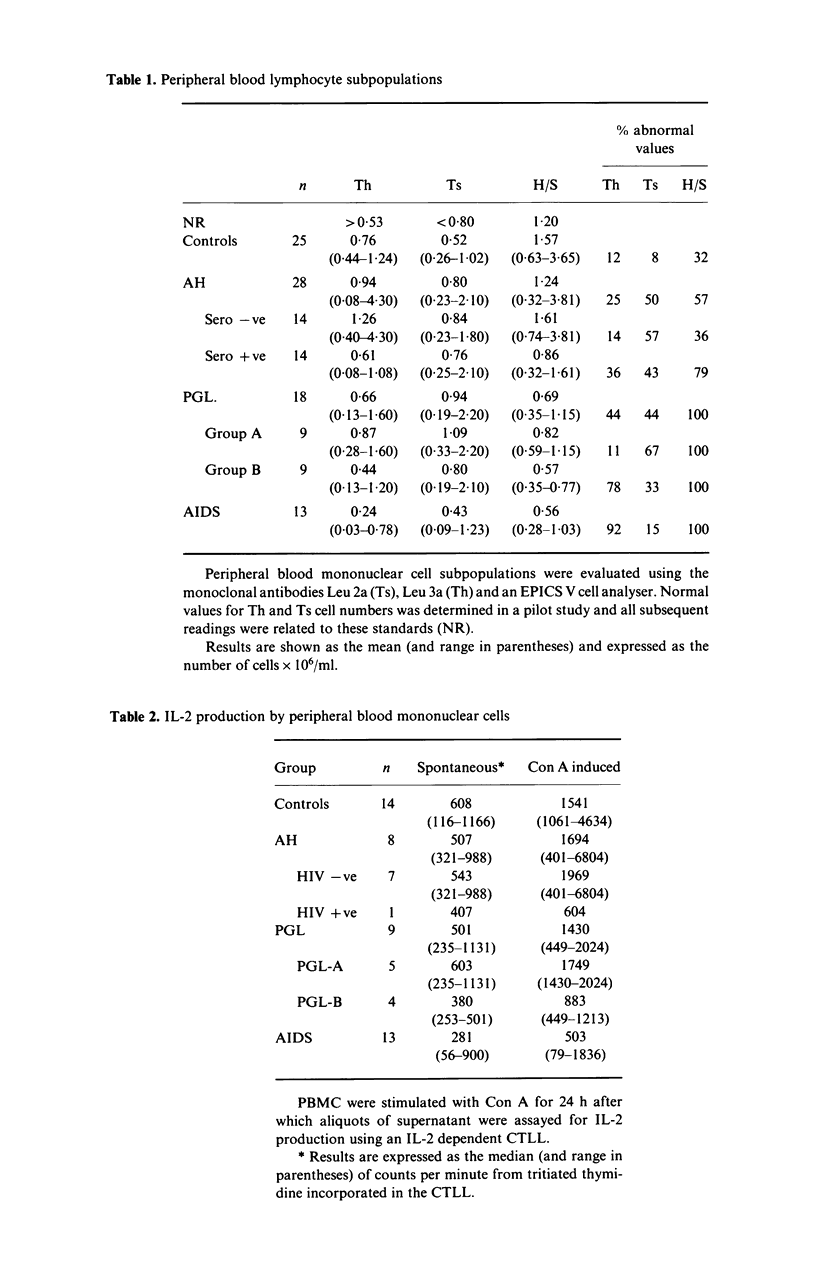
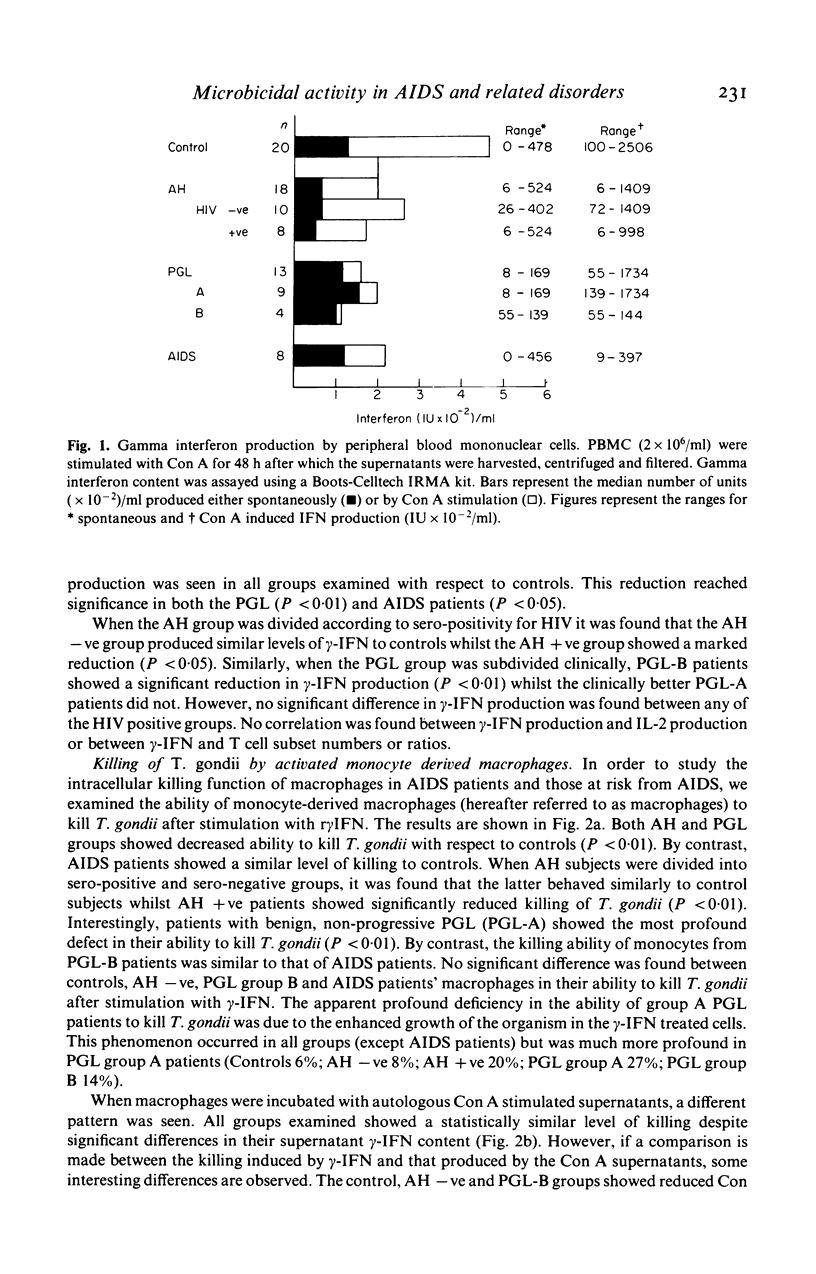
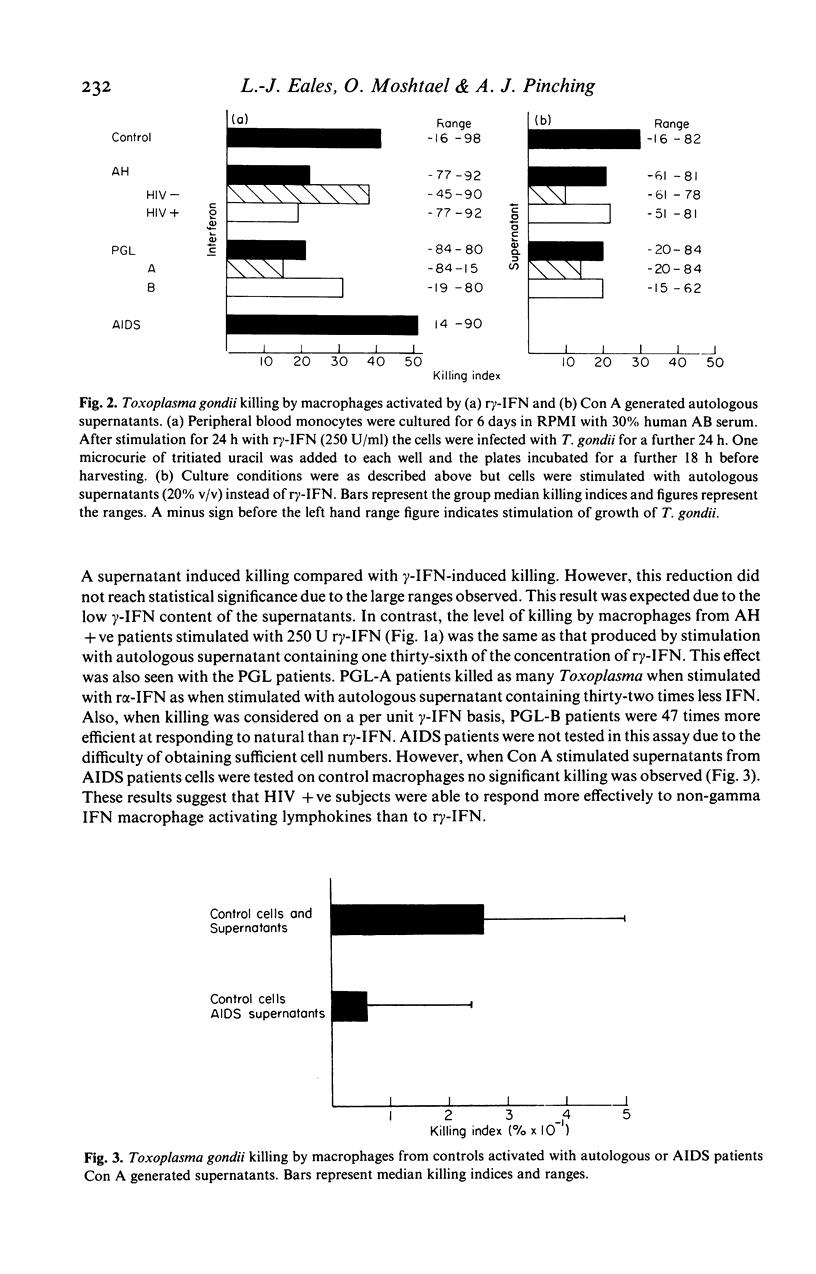
We have examined the ability of monocyte-derived macrophages from patients with AIDS and other HIV-related disorders to kill the intracellular pathogen Toxoplasma gondii. We have also examined the capacity of peripheral blood mononuclear cells from these patients to produce macrophage-activating and other lymphokines. The capacity to produce interleukin 2 and gamma interferon decreases from controls through asymptomatic seropositive subjects and lymphadenopathy groups A (benign) and B (prodromal) to AIDS. The decrease did not correlate precisely with the decrease in CD4+ cells in these patients. Monocyte-derived macrophages from asymptomatic HIV-infected subjects and lymphadenopathy patients showed a decreased ability to kill T. gondii after activation with recombinant gamma interferon; paradoxically, this was most striking for PGL group A. The defect was largely overcome by using Concanavalin A stimulated autologous supernatants. It was notable that macrophages from AIDS patients showed normal killing with recombinant gamma interferon, but that the supernatants from AIDS patients had reduced activity with normal macrophages. These studies confirm that functional defects of both lymphocytes and macrophages are found in HIV-infected subjects; they serve to emphasize the heterogeneity of the clinical and biological responses to this retrovirus, responses which have important implications in the pathogenesis and treatment of the immunodeficiency.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcocer-Varela J., Alarcon-Segovia D., Abud-Mendoza C. Immunoregulatory circuits in the acquired immune deficiency syndrome and related complex. Production of and response to interleukins 1 and 2, NK function and its enhancement by interleukin-2 and kinetics of the autologous mixed lymphocyte reaction. Clin Exp Immunol. 1985 Apr;60(1):31–38. [PMC free article] [PubMed] [Google Scholar]
- Anderson S. E., Jr, Remington J. S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974 May 1;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew P. W., Rees A. D., Scoging A., Dobson N., Matthews R., Whittall J. T., Coates A. R., Lowrie D. B. Secretion of a macrophage-activating factor distinct from interferon-gamma by human T cell clones. Eur J Immunol. 1984 Oct;14(10):962–964. doi: 10.1002/eji.1830141018. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Buimovici-Klein E., Lange M., Ramey W. G., Grieco M. H., Cooper L. Z. Cell-mediated immune responses in AIDS. N Engl J Med. 1984 Aug 2;311(5):328–329. doi: 10.1056/NEJM198408023110512. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Sarin P. S., Gelmann E. P., Robert-Guroff M., Richardson E., Kalyanaraman V. S., Mann D., Sidhu G. D., Stahl R. E., Zolla-Pazner S. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):865–867. doi: 10.1126/science.6601823. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Lopez D., Husmann L., Meyer P. R., Taylor C. R. Heterogeneity of macrophage populations in human lymphoid tissue and peripheral blood. Cell Immunol. 1984 Oct 1;88(1):61–74. doi: 10.1016/0008-8749(84)90052-2. [DOI] [PubMed] [Google Scholar]
- Hofmann B., Odum N., Platz P., Ryder L. P., Svejgaard A., Neilsen J. O. Immunological studies in acquired immunodeficiency syndrome. Functional studies of lymphocyte subpopulations. Scand J Immunol. 1985 Mar;21(3):235–243. doi: 10.1111/j.1365-3083.1985.tb01426.x. [DOI] [PubMed] [Google Scholar]
- Kasahara T., Hooks J. J., Dougherty S. F., Oppenheim J. J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983 Apr;130(4):1784–1789. [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Depper J. M., Greene W. C., Whalen G., Waldmann T. A., Fauci A. S. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N Engl J Med. 1985 Jul 11;313(2):79–84. doi: 10.1056/NEJM198507113130204. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Edgar L. C., Whalen G., Rook A. H., Fauci A. S. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983 Aug 25;309(8):453–458. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- Lin R. Y., Tanz W. S., Denny T. N. Suppressor function of T lymphocytes in the acquired immunodeficiency syndrome as assessed by allogeneic mixed lymphocyte culture. J Clin Lab Immunol. 1985 Feb;16(2):69–73. [PubMed] [Google Scholar]
- McLeod R., Remington J. S. A method to evaluate the capacity of monocytes and macrophages to inhibit multiplication of an intracellular pathogen. J Immunol Methods. 1979 May 10;27(1):19–29. doi: 10.1016/0022-1759(79)90235-7. [DOI] [PubMed] [Google Scholar]
- Moretta A. Frequency and surface phenotype of human T lymphocytes producing interleukin 2. Analysis by limiting dilution and cell cloning. Eur J Immunol. 1985 Feb;15(2):148–155. doi: 10.1002/eji.1830150208. [DOI] [PubMed] [Google Scholar]
- Moscicki R. A., Amento E. P., Krane S. M., Kurnick J. T., Colvin R. B. Modulation of surface antigens of a human monocyte cell line, U937, during incubation with T lymphocyte-conditioned medium: detection of T4 antigen and its presence on normal blood monocytes. J Immunol. 1983 Aug;131(2):743–748. [PubMed] [Google Scholar]
- Murray H. W., Gellene R. A., Libby D. M., Rothermel C. D., Rubin B. Y. Activation of tissue macrophages from AIDS patients: in vitro response of AIDS alveolar macrophages to lymphokines and interferon-gamma. J Immunol. 1985 Oct;135(4):2374–2377. [PubMed] [Google Scholar]
- Murray H. W., Hillman J. K., Rubin B. Y., Kelly C. D., Jacobs J. L., Tyler L. W., Donelly D. M., Carriero S. M., Godbold J. H., Roberts R. B. Patients at risk for AIDS-related opportunistic infections. Clinical manifestations and impaired gamma interferon production. N Engl J Med. 1985 Dec 12;313(24):1504–1510. doi: 10.1056/NEJM198512123132403. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Masur H., Roberts R. B. Impaired production of lymphokines and immune (gamma) interferon in the acquired immunodeficiency syndrome. N Engl J Med. 1984 Apr 5;310(14):883–889. doi: 10.1056/NEJM198404053101404. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Leonard E. J., Meltzer M. S. Macrophages in resistance to rickettsial infections: characterization of lymphokines that induce rickettsiacidal activity in macrophages. J Immunol. 1981 Jan;126(1):204–207. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinching A. J., McManus T. J., Jeffries D. J., Moshtael O., Donaghy M., Parkin J. M., Munday P. E., Harris J. R. Studies of cellular immunity in male homosexuals in London. Lancet. 1983 Jul 16;2(8342):126–130. doi: 10.1016/s0140-6736(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Poli G., Bottazzi B., Acero R., Bersani L., Rossi V., Introna M., Lazzarin A., Galli M., Mantovani A. Monocyte function in intravenous drug abusers with lymphadenopathy syndrome and in patients with acquired immunodeficiency syndrome: selective impairment of chemotaxis. Clin Exp Immunol. 1985 Oct;62(1):136–142. [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Tzeng D. Y., Deuel T. F., Huang J. S., Baehner R. L. Platelet-derived growth factor promotes human peripheral monocyte activation. Blood. 1985 Jul;66(1):179–183. [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]


