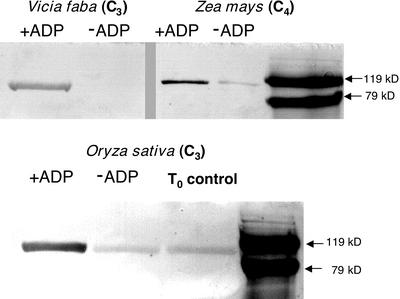
Figure 7.
Purified maize PPDK is phosphorylated in vitro by an ADP-requiring activity present in desalted leaf extracts of V. faba and rice. The immunoblot-based, in vitro RP-phosphorylation assays were initiated by combining aliquots of desalted leaf extracts from maize, V. faba, or rice with purified maize recombinant (non-phospho) PPDK in the presence or absence of 1 mm ADP. Aliquots of the in vitro reactions, containing either 1.75 μg of PPDK with rice and V. faba or 90 ng for maize were electrophoresed in SDS-PAGE gels, and the resulting immunoblots probed with phospho-PPDK antibody. Visible bands indicate the position of phospho-PPDK corresponding to the ≈95-kD PPDK monomer as estimated by molecular mass standards on the same blot. The T0 control in the rice blot represents endogenous phospho-PPDK present in the dark-leaf extract before initiating the RP assay. Likewise, in the rice and maize −ADP lanes, the faint band of phospho-PPDK is the result of carryover of endogenous phospho-PPDK present in the aliquot of the dark-leaf extract used for the in vitro RP assay (Chastain et al., 2000).