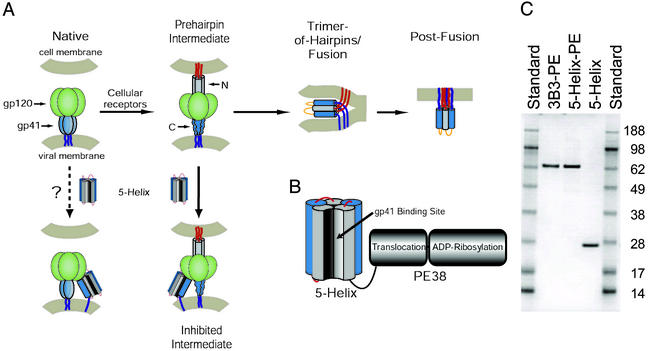
Figure 1.
Targeting therapeutics to the HIV-1 fusion protein. (A) Schematic of HIV-1 Env and working model of membrane fusion. The binding of gp120 to cellular receptors triggers gp41 to extend and insert its N-terminal fusion peptide into the target cell membrane (10). In this prehairpin intermediate, the N-terminal coiled coil is exposed and can be targeted by C-peptides. In the absence of inhibitor, this transient state ultimately collapses into a trimer-of-hairpins that brings the N- and C-terminal regions into close proximity, promoting membrane fusion. The entry inhibitor 5-Helix is designed to target the C-terminal region of the gp41 ectodomain and prevent the formation of the gp41 trimer-of-hairpins. The coloring scheme for gp41 is dark blue for the transmembrane sequence, light blue for the C-terminal region, gray for the N-terminal region, and red for the N-terminal fusion peptide; gp120 is colored green. (B) Design of 5-Helix-PE. The 5-Helix domain contains five of the six α-helices that constitute the gp41 trimer-of-hairpins linked together into a single polypeptide. Its C terminus is connected with a 13-aa linker to the translocation and ADP-ribosylation domains of PE38. (C) SDS-gel analysis of purified 3B3-PE, 5-Helix-PE, and 5-Helix. One microgram of each protein was analyzed by SDS/PAGE alongside SeeBlue Plus2 prestained protein standards (Invitrogen). Predicted molecular masses are 65.1 kDa for 3B3-PE, 64.6 kDa for 5-Helix-PE, and 25.4 kDa for 5-Helix.