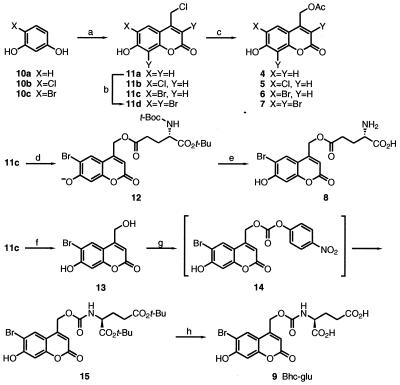
Figure 2.
Synthetic scheme for the new compounds prepared in this study. (a) Ethyl 4-chloroacetoacetate, H2SO4, rt, 6 d; (b) Br2, AcOH, rt, 1h; (c) DBU, AcOH, benzene, reflux, 1.5 h; (d) DBU, N-(tert-butoxycarbonyl)-α-tert-butyl glutamate, benzene, reflux, 1 h; (e) CF3CO2H, CH2Cl2, rt, 1 d; (f) H2O, reflux, 14 h; (g) i. DMAP, 4-nitrophenyl chloroformate, CH3CN, rt, 7 h. ii DMAP, di-tert-butyl glutamate hydrochloric acid salt, rt, 23 h; (h) CF3CO2H, CH2Cl2, rt, 2 d.