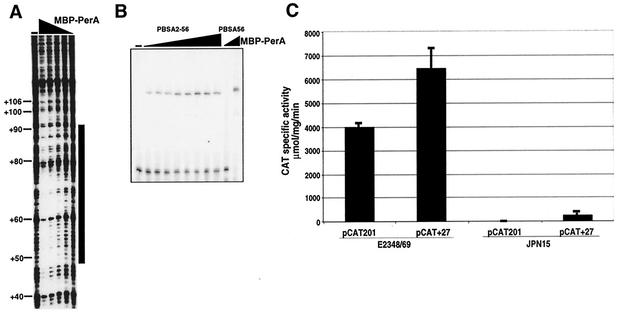
FIG. 7.
Characterization of the second binding site on bfpA. A) The DNase I protection assay of a fragment containing the sequence from positions −54 to +166 was carried out as described in the legend to Fig. 4. The black triangle above the lanes indicates decreasing amounts of MBP-PerA (0, 2.6 μM, 1.7 μM, 880 nM, 528 nM, 176 nM, and 88 nM). (B) EMSA of a 56-mer double-stranded oligonucleotide containing the bfpA sequence between positions +38 and +93 (PBSA2-56) (Table 2). The triangle above the lanes indicates increasing amounts of MBP-PerA (0, 133 nM, 238 nM, 475 nM, 958 nM, 1.85 μM, 5.04 μM, 6.6 μM, and 8.4 μM). Probe PBSA56 was included as a control in the absence or presence of 1.85 μM MBP-PerA. (C) The second PerA binding sequence is not required for bfpA expression. EPEC strains E2348/69 (wild type) and JPN15 (pEAF cured) were transformed with either pCAT201 or pCAT + 27, which carry transcriptional fusions between the reporter cat gene and bfpA regulatory fragments from positions −201 to +76 and −201 to +27, respectively. The CAT specific activity was determined from samples collected from bacterial cultures grown on DMEM at an OD600 of 1.0. The results are the average of three different experiments.