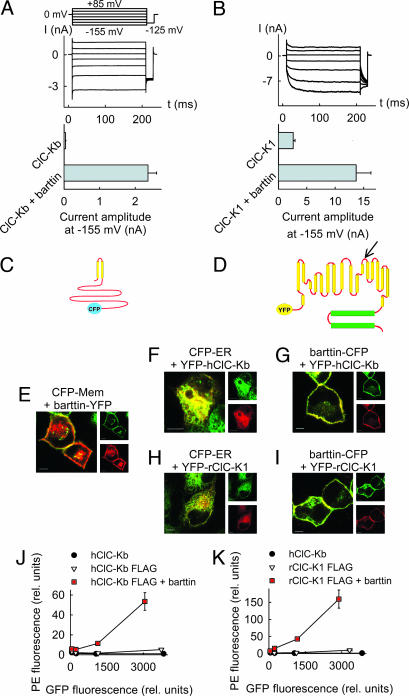
Fig. 1.
Barttin promotes surface membrane insertion of ClC-K channels. (A and B) Representative currents from cells coexpressing hClC-Kb (A) or rClC-K1 (B) with barttin and mean isochronal current amplitudes determined 2 ms after a voltage step to −155 mV (n > 8) from cells expressing channels with and without barttin. (C and D) Topology model of barttin (C) and ClC-K channels (D). The position of the FLAG epitope used for FACS analysis is marked by an arrow. (E) Confocal images of live MDCK cells coexpressing a barttin-YFP fusion protein and a fluorescent marker for the membrane surface (CFP-Mem; Clontech). CFP is shown in green, and YFP is shown in red. This color code results in an orange coloring of regions where both proteins overlap. (F and H) Confocal images of live MDCK cells coexpressing a fluorescent marker for the endoplasmic reticulum (CFP-ER; Clontech) together with YFP-hClC-Kb (F) and YFP-rClC-K1 (H). (G and I) Confocal images of live MDCK cells coexpressing a barttin-CFP fusion protein and YFP-hClC-Kb (G) or YFP-rClC-K1 (I). (Scale bars: 5 μm.) (J and K) Surface expression of hClC-Kb (J) and rClC-K1 (K) with (■) and without (▿) barttin in transiently transfected tsA201 cells determined by flow cytometry. Shown are plots of fluorescence levels of an anti-Flag antibody versus the GFP fluorescence. Data were binned in four groups where means and SEMs were obtained. GFP-hClC-Kb without FLAG (●) was used as a control. For both channels, antibody fluorescence is significantly different from control level (P < 0.05), and barttin significantly increases the fluorescence levels (P < 0.05).