Fig. 7.
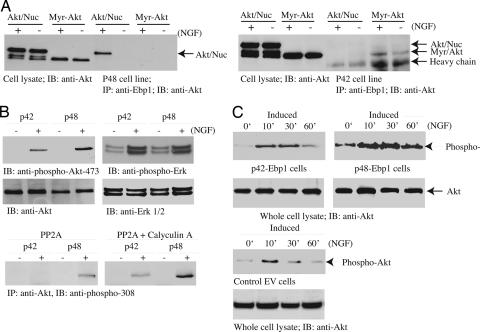
p48 selectively binds nuclear Akt and sustains its kinase activity. (A) p48 but not p42 strongly interacts with nuclear Akt. p48 and p42 stable cell lines were induced in the medium without tetracycline for 24 h, infected with adenovirus expressing myristolated-Akt or nuclear Akt, and stimulated with NGF for 30 min. Ebp1 was immunoprecipitated and its associated proteins were analyzed with anti-Akt antibody. (Left) NGF stimulated nuclear but not cytoplasmic Akt to bind p48. (Right) By contrast, NGF enhanced cytoplasmic but not nuclear Akt to interact with p42. (B) Akt activation is stronger in p48 but not in p42 cells. p48 and p42 stable cell lines were induced and treated with NGF for 30 min. Cell lysate was monitored by immunoblotting with anti-phospho-Akt-473, anti-Akt, anti-phospho-Erk, and anti-Erk1/2 antibodies. Akt was immunoprecipitated from Ebp1 cells and incubated with purified active PP2A (1 unit, 0.5 μg) at 30°C for 1 h in the presence or absence of calyculin A. PP2A dephosphorylated Akt immunoprecipitated from p42 but not p48 cells, which can be inhibited by phosphatase inhibitor calyculin A (10 μM) pretreatment. (C) Akt activation was sustained and stronger in p48 cells compared with control and p42 cells.