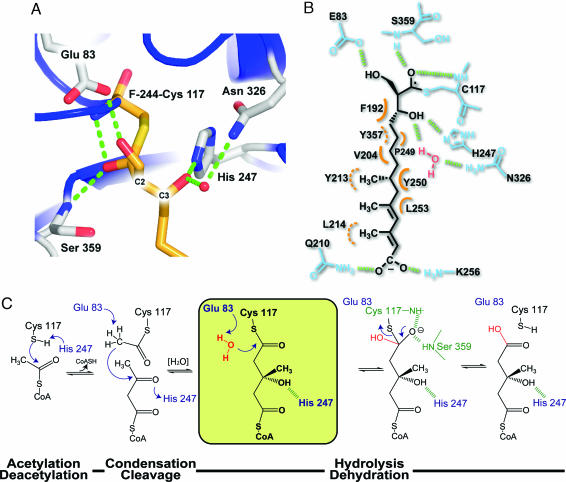
Fig. 4.
Close-up view of F-244 covalently modifying the BjHMGS1 active site and a posited reaction mechanism based on the F-244 complex. (A) Secondary structure is shown as ribbons colored as in Fig. 1. Side chains and F-244 are depicted as half-colored bonds with red for oxygen, blue for nitrogen, and gold and gray for carbons on F-244 and HMGS side chains, respectively. The oxygen atoms of the ring-opened form of F-244 form H-bonds (green dashes) with Glu-83, His-247, and Asn-326 through a water molecule (red sphere) and backbone amides of Ser-359 and Cys-117. (B) Schematic representation of F-244 tethered to Cys-117 highlighting all intermolecular interactions. H-bonds are depicted as green dashes. Orange half circles depict van der Waals contacts with dashed curves specifying residues sitting behind the plane formed by F-244. (C) Putative reaction mechanism of HMGS. The proposed role of Glu-83 in the last hydrolytic step necessary for release of HMG-CoA is shown in a yellow box. The residues implicated in formation of the oxyanion hole are depicted in green, and H-bonds are shown as dashed bonds.