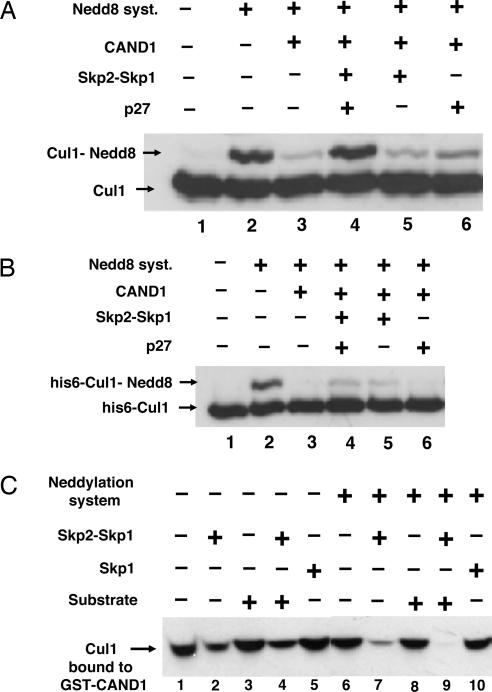
Fig. 2.
Skp2 causes dissociation of Cul1 from CAND1 and, jointly with substrate, abrogates inhibition of neddylation. (A) Skp2 and substrate overcome inhibition by CAND1 of the neddylation of Cul1 in a partially purified preparation. Assay of the neddylation of Cul1 was carried out as described in Materials and Methods in the presence of 0.5 μl of fraction 9 of the heparin column as the source of Cul1. In lanes 3 and 5, a substrate mixture lacking p27 was added. Where indicated, CAND1 (70 nM) was added 5 min before the neddylation mixture. (B) Neddylation of purified recombinant Cul1 in the presence of CAND1 is only slightly stimulated by Skp2–Skp1. Experimental conditions were as in A, except that partially purified Cul1 was replaced by 35 nM recombinant purified His-6-Cul1-Roc1. (C) Skp2–Skp1, but not Skp1 or substrate, promotes dissociation of Cul1–Cand1 complex. Bacterially expressed GST–CAND1 (1.3 pmol) was preincubated with 4 μl of pooled fractions 10–12 from the heparin-Sepharose column for 30 min at 30°C in a buffer consisting of 50 mM Tris·HCl, pH 7.6/5 mM MgCl2/1 mM DTT/10% glycerol/10 mM phosphocreatine/0.1 μg/μl creatine phosphokinase/0.5 mM ATP/3 mg/ml BSA. Subsequently, Skp2–Skp1 (10 nM), Skp1 (15 nM), or complete substrate mixture (see Materials and Methods) was added as indicated, and a second incubation (at 20°C for 15 min) was carried out under conditions as described for the assay of neddylation of Cul1, except that the reaction volume was 40 μl and a neddylation system (consisting of APP-BP1-Uba3, Ubc12, and Nedd8) was added only in the indicated tubes. Next, 10 μl of glutathione-Sepharose beads (Amersham Pharmacia Biosciences) was added, and samples were rotated at 4°C for 90 min. The beads were washed three times with a buffer consisting of 50 mM Tris·HCl, pH 7.2/1 mM DTT/2 mg/ml BSA/100 mM NaCl/10% glycerol, eluted with SDS sample buffer, and subjected to immunoblotting for Cul1 for the estimation of Cul1 that remained bound to GST–CAND1.