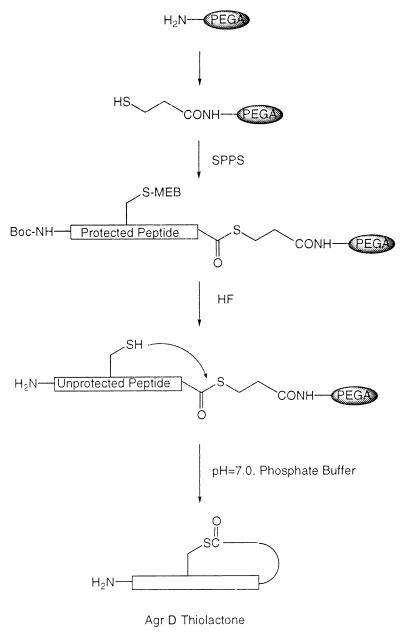
Figure 1.
Chemical synthesis of AgrD thiolactone-containing autoinducing peptides via a solid-phase intramolecular chemical ligation strategy. Key to this process is the ability to prepare a fully unprotected peptide immobilized on a solid support through a reactive thiol ester bond. Simply swelling such an unprotected peptide-[thioester]-resin in aqueous buffer results in a chemoselective ligation reaction and concomitant cleavage of the peptide from the support. This final step is made possible because of the excellent swelling properties of the PEGA support in water (21).