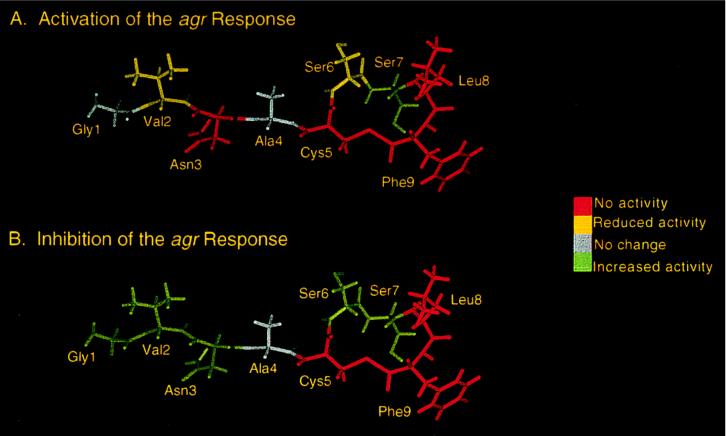
Figure 4.
Effect of replacing each residue within the AgrDII peptide sequence with alanine on activation (A) and inhibition (B) activity. The results are superimposed on a model of the AgrDII peptide created by using the program discover (Biosym Technologies, San Diego). The ED50 values (activation, group II cells) were as follows: Ala-1, 3.2 ± 2.2 nM; Ala-2, 73.7 ± 1.9 nM; Ala-3, no activity; Ala-6, 33.6 ± 1.5 nM; Ala-7, <1 nM; Ala-8, no activity; Ala-9, no activity. The IC50 values (inhibition, group I cells) were as follows: Ala-1, <1 nM; Ala-2, <1 nM; Ala-3, <1 nM; Ala-6, ≪1 nM; Ala-7, <1 nM; Ala-8, no activity; Ala-9, no activity. None of the peptides activated the agr response in group I S.aureus strains or inhibited the agr response in group II strains.