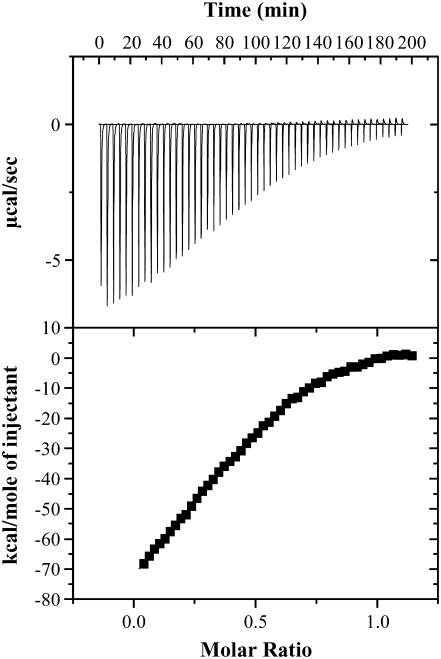
FIGURE 10.
Binding isotherm representing the titration of apo-S100A13 with the C2A domain. S100A13 and the C2A domain were prepared in 25 mM Tris buffer (pH 7.2), containing 25 mM KCl. The apparent dissociation constant defining the affinity of the apo-S100A13 to the C2A domain is estimated to be in the micromolar range. ITC experiments were performed at 25°C and the data were corrected for heats of dilution and ionization. The upper and lower panels represent the raw data and the best fits of the raw data, respectively.