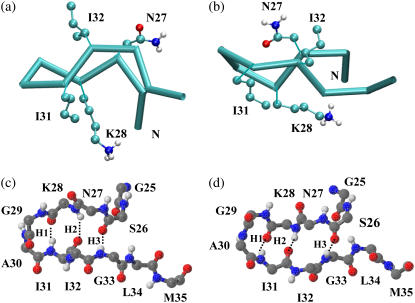
FIGURE 4.
Representative structures of the two most populated β-hairpin conformations. Both possess a type II′ β-turn involving residues G29 and A30 and two short β-strands involving residues N27, K28, I31, and I32, but differ in the relative twist of the strands. A trace representation of the β-hairpins and a CPK representation of the side chains of the β-stranded residues are shown in panels a and b. Structures a and b are located at positions (0.42 nm, 0.03 nm) and (0.44 nm, 0.19 nm), respectively, in Fig. 2. A fully atomic representation of the main chain of the two β-hairpins is shown in panel c (the same conformation as shown in a) and panel d (the same conformation as shown in b). H-bonds are represented by dotted lines and are numbered from the turn to the tail of the β-hairpin (H1–H3). The two β-hairpins have the same H-bond pattern. The N-terminus of the peptide is labeled with N.