Fig. 3.
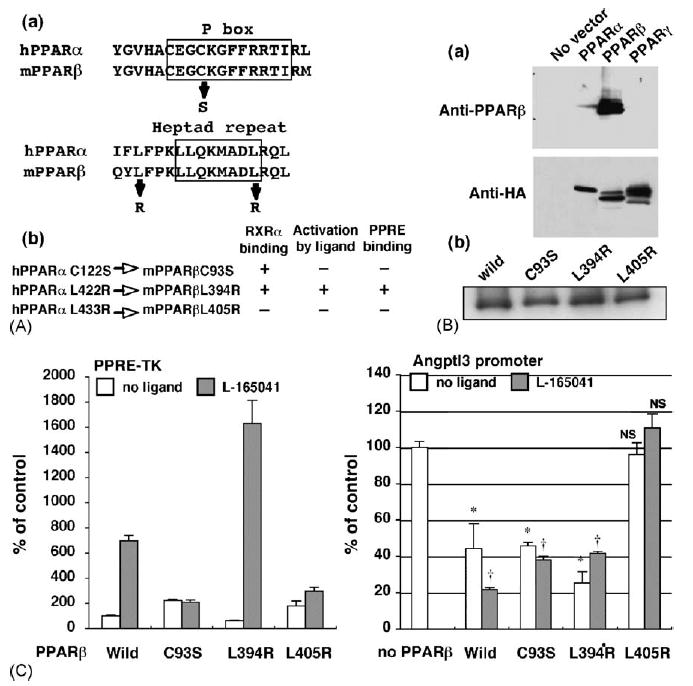
PPARβ mutant lacking RXRα binding capacity has no effect on the angptl3 promoter activity. (A-a) The human PPARα sequences of the P box region and heptad repeat motif were aligned with mouse PPARα. The substituted amino acids are shown as arrows. (A-b) Characterization of each PPARβ point mutant. The amino residues mutated in human PPARα protein were completely conserved in mouse PPARβ. These mutants were characterised in an earler report (Juge-Aubry et al., 1995). (B-a) Each PPAR isoform expression vector containing C terminal HA epitop tag was transfected to HEK293 cells. Western blot using the cell lysates was performed by PPARβ (upper panel) or HA (lower panel) antibody. (B-b) The same levels of full-length PPARβ wild-type and each mutant protein expression were observed after transient expression in HEK293 cells as assayed by Western blotting. (C) The effect of mutanted PPARβ on the PPRE-TK (left) and angptl3 (right) promoter activity. HepG2 cells were transfected with the angptl3 promoter (D4 mutant as shown in Fig. 2A) or thymidine kinase promoter including the PPRE sequence plasmids. Cells were treated with or without 1 μM L-165041 and 100 nM T0901317 (when the angptl3 promoter was used). The results are displayed as % values of PPARβ wild-type vector without L-165041 (PPRE-TK) or without L-165041 and PPARβ vector (angpyl promoter). Significant differences from control (without PPARβ): *p < 0.001; †p < 0.001; NS, no significant difference.
