Fig. 7.
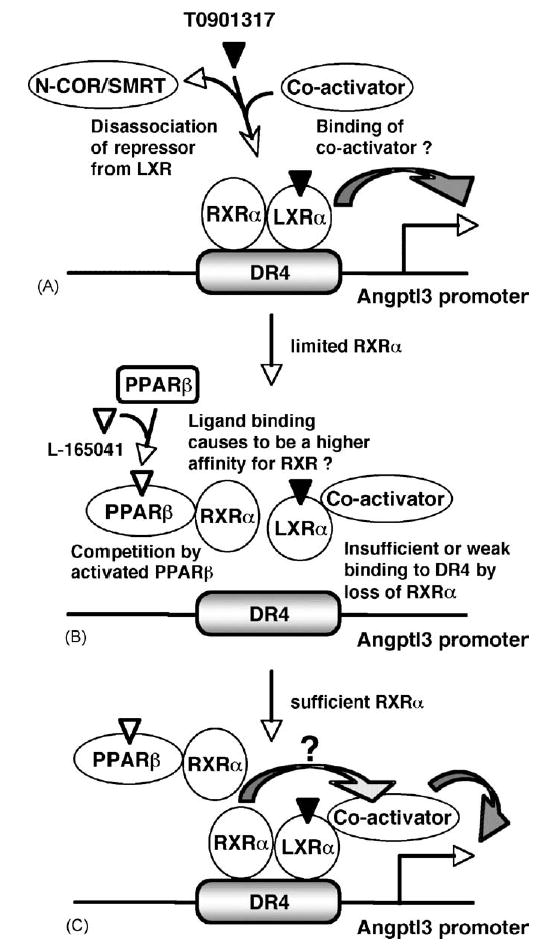
A simplified repression model of angptl3 promoter activity by PPARβ. (A) LXRα regulates the angptl3 promoter activity (Inaba et al., 2003; Kaplan et al., 2003). RXRα facilitates binding of type 2 nuclear receptors to their response elements. Activation by LXRα ligand disassociates repressors N-COR/SMART (Hu et al., 2003) and promotes association with co-activator (Mangelsdorf and Evans, 1995). (B) PPARβ is also a partner for RXRα. Therefore, activated PPARβ interfers the binding of LXRα to the LXR response element DR4 in the angptl3 promoter. L165041-activated PPARβ may result in higher affinity binding to RXRα. (C) Increased cellular levels of RXRα overcomes the inhibition by PPARβ leading to sufficient LXRα/RXRα for binding to the DR4. Thus, RXRα not only facilitates binding to the DR4 element but also may contribute to the additive effect of LXRα-transcriptional activity through an unknown pathway.
