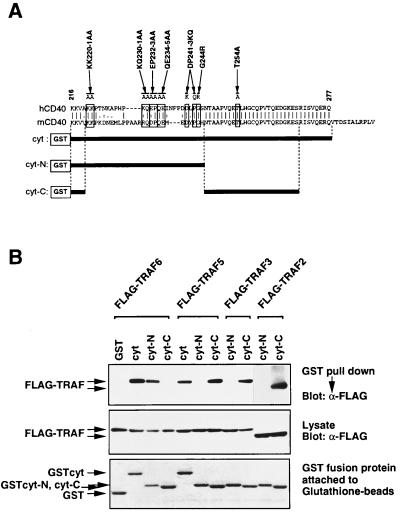
Figure 1.
TRAF2, TRAF3, and TRAF5 bind to the carboxyl-terminal conserved domain, but TRAF6 binds to the N-terminal domain in CD40cyt. (A) Alignment of human and mouse CD40cyt. The carboxyl-terminal part of CD40cyt is identical between hCD40 and mCD40, but the N-terminal part is poorly conserved. Conserved residues substituted in mutagenesis described in the text are indicated by boxes, and the resulting aas by the mutagenesis are shown above these boxes. GST-tagged hCD40cyt constructs (cyt, cyt-N and cyt-C) are shown below the sequences. (B) In vivo association of TRAFs with GST-hCD40 in 293T cells. Cell extracts from 293T cells cotransfected with expression plasmids for Flag-tagged TRAFs and GST-tagged hCD40cyt or its deletion mutants were prepared. The GST pulldown assays were performed and TRAF proteins bound to beads were then analyzed by Western blotting by using anti-Flag antibody M2 (Top). One-tenth of GST fusion proteins attached to beads was separated on 12.5% polyacrylamide/SDS gel and visualized by Coomassie brilliant blue R-250 staining (Bottom). A portion of the lysate used for the GST pulldown assay was separated on 8.5% polyacrylamide/SDS gel and expression level of Flag-tagged TRAFs was analyzed by Western blotting by using anti-Flag antibody M2 (Middle).