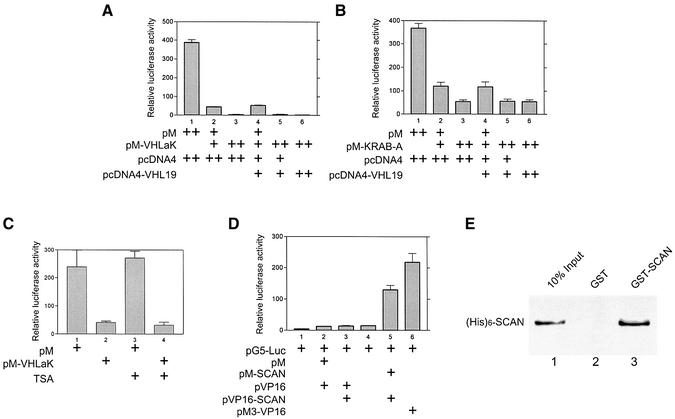
Fig. 3. The KRAB-A domain of VHLaK exhibits transcriptional repression activity independent of HDAC activity and the homo-oligomerization mediated by the SCAN domain enhances this repressive activity. (A and B) Transcriptional repression activity of the VHLaK protein and its KRAB-A domain. Various doses of GAL4-DBD-VHLaK (pM-VHLaK) (A) and of GAL4-DBD-KRAB-A (pM-KRAB) (B), along with pVHL expression plasmid (pcDNA4-VHL19), 300 ng of pGAL4-tk-LUC and 10 ng pRLSV40-LUC, were transfected into COS-7 cells. Twenty-four hours after transfection, cells were harvested and measured for firefly and Renilla activity. The firefly luciferase activity was normalized against Renilla luciferase activity for each sample, and mean values of relative luciferase activity were calculated from triplicate wells. The experiments were repeated three times to ensure consistency. (C) Transcriptional repression activity of VHLaK protein is independent of HDAC activity. Lanes 3 and 4, 18 h after transfection, the cells were treated with 300 nM of trichostatin A (TSA) for another 18 h and then the cells were lysed for luciferase activity assay. (D) Analysis of self-interaction of the SCAN domain in mammalian two-hybrid assay. Lane 5, pM-SCAN and pVP16-SCAN were co-transfected into COS-7 cells together with reporter plasmid pG5-LUC and cells were lysed for luciferase activity assay. Lane 6, pM3-VP16 served as a positive control. (E) Analysis of the self-interaction of the SCAN domain by GST pull-down assay. Five microliters of in vitro translated [35S]methionine-labeled His6-SCAN protein were incubated with GST–SCAN or GST-coated beads. The eluted solutions and 10% of input protein were resolved on a 15% SDS–PAGE gel.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.