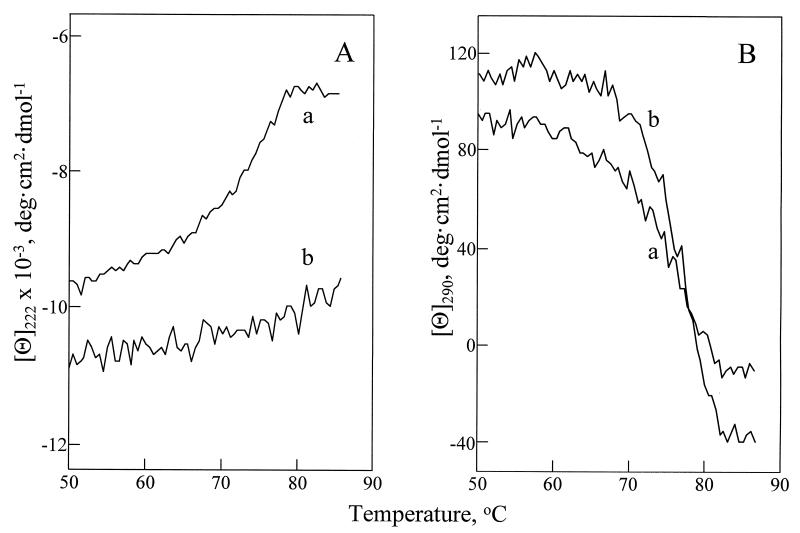
Figure 2.
Temperature melting of the secondary (A) and tertiary (B) structure of lysozyme in water at pH 3.8 (a) and in glycerol (b). The lyophilized protein was dissolved in water (pH 3.8) at 12 mg/ml, filtered, and diluted 1:100 with either water (pH 3.8) or glycerol. Unfolding of the secondary and tertiary structures of lysozyme was followed by measuring the changes in the mean residue ellipticity. The heating rate was 20°C/hr. Mean residue ellipticities of water at pH 3.8 and of glycerol containing 1% water (pH 3.8) were examined separately as a function of temperature and subtracted for baseline corrections. The Tm values of lysozyme’s unfolding were determined as described in ref. 12.