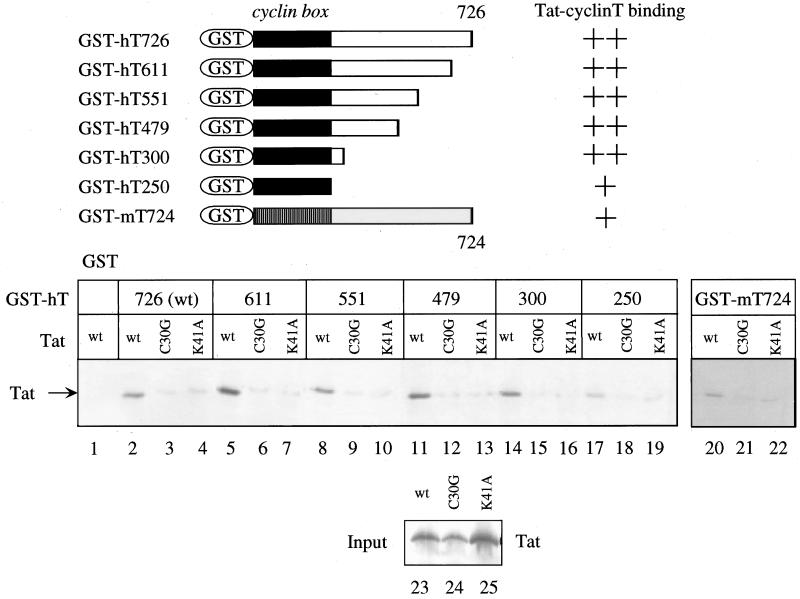
Figure 2.
N-terminal 300 residues in human cyclin T mediate its specific binding to Tat, and mouse cyclin T binds weakly to Tat. Wild-type and mutant 35S-labeled Tat proteins were incubated with GST and hybrid GST-cyclin T proteins and were selected on glutathione-Sepharose beads. Bound proteins were revealed by SDS/PAGE followed by autoradiography. Besides full length human [GST-hT726, wild-type (wt)] and mouse cyclin T proteins (GST-mT724), C-terminal truncations of human cyclin T to positions 611 (GST-hT611), 551 (GST-hT551), 479 (GST-hT479), 300 (GST-hT300), and 250 (GST-hT250) also were examined. These truncations and the summary of the binding data are depicted on the top. Mutant Tat proteins contained a substitution of the cysteine to glycine at position 30 (TatC30G) and of the lysine to alanine at position 41 (TatK41A) in the activation domain of Tat, which render Tat inactive. The input of Tat, mutant Tat proteins, and chimeras was equal in all reactions (lanes 23 to 25).