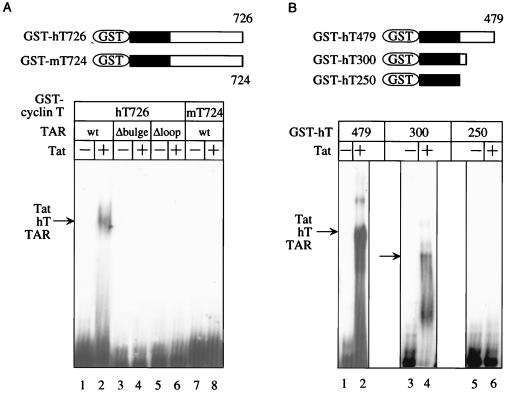
Figure 3.
Analysis of interactions between cyclin T proteins, Tat and TAR. (A) Mouse cyclin T does not support interactions between Tat and TAR in vitro. The hybrid GST-human cyclin T (hT726) and hybrid GST-mouse cyclin T (mT724) proteins were incubated with 32P-labeled TAR [lanes 1, 2, 7, and 8, wild-type (wt)], TAR lacking the 5′ bulge (lanes 3 and 4, Δbulge), or TAR lacking the central loop (lanes 5 and 6, Δloop). Tat was added to reactions in lanes 2, 4, 6, and 8. RNA–protein complexes were resolved by a 6% nondenaturing polyacrylamide gel. (B) N-terminal 300 residues in human cyclin T support interactions between Tat and TAR in vitro. Human cyclin T proteins, which were truncated from the C terminus to position 479, 300, and 250 (hT479, hT300, and hT250), were incubated with TAR. Tat was added to the reaction in lanes 2, 4, and 6. RNA–protein complexes are indicated by arrows. A schematic representation of cyclin T proteins used in these reactions is given on the top.