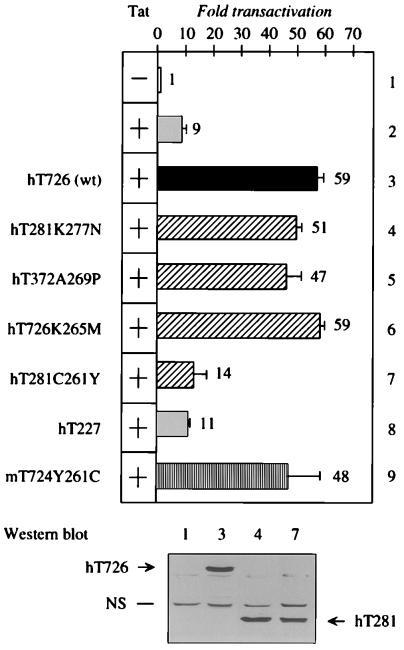
Figure 4.
N-terminal 281 residues in human cyclin T support Tat transactivation. Reciprocal substitutions of the cysteine and tyrosine at position 261 inactivate human cyclin T and activate mouse cyclin T, respectively, in CHO cells. The HIV-1 long terminal repeat was expressed alone [pHIVSCAT (lane 1)] or together with Tat [pcDNA3Tat (lane 2)]. To Tat were added human cyclin T [hT726 (lane 3)], truncated human cyclin T containing the substitution of lysine to asparagine at position 277 [hT281K277N (lane 4)], truncated human cyclin T containing the substitution of the alanine to proline at position 269 [hT372A269P (lane 5)], human cyclin T containing the substitution of the lysine to methionine at position 265 [hT726K265M (lane 6)], truncated human cyclin T containing the substitution of the cysteine to tyrosine at position 261 [hT281C261Y (lane 7)], truncated human cyclin T to position 227 [hT227 (lane 8)], and mouse cyclin T containing the substitution of the tyrosine at position 261 to cysteine [mT724Y261C (lane 9)]. Solid and striped bars denote wild-type or truncated and mutant cyclin T proteins, respectively. The value of the CAT activity of pHIVSCAT alone was set to 1. Standard errors of the mean from three independent transfections are presented. Western blotting revealed that levels of expression of the full length and truncated human cyclin T proteins (hT281K277N and hT281C261Y) were similar. Numbers above the lanes correspond to lanes from coexpression assays. NS, nonspecific bands.