Abstract
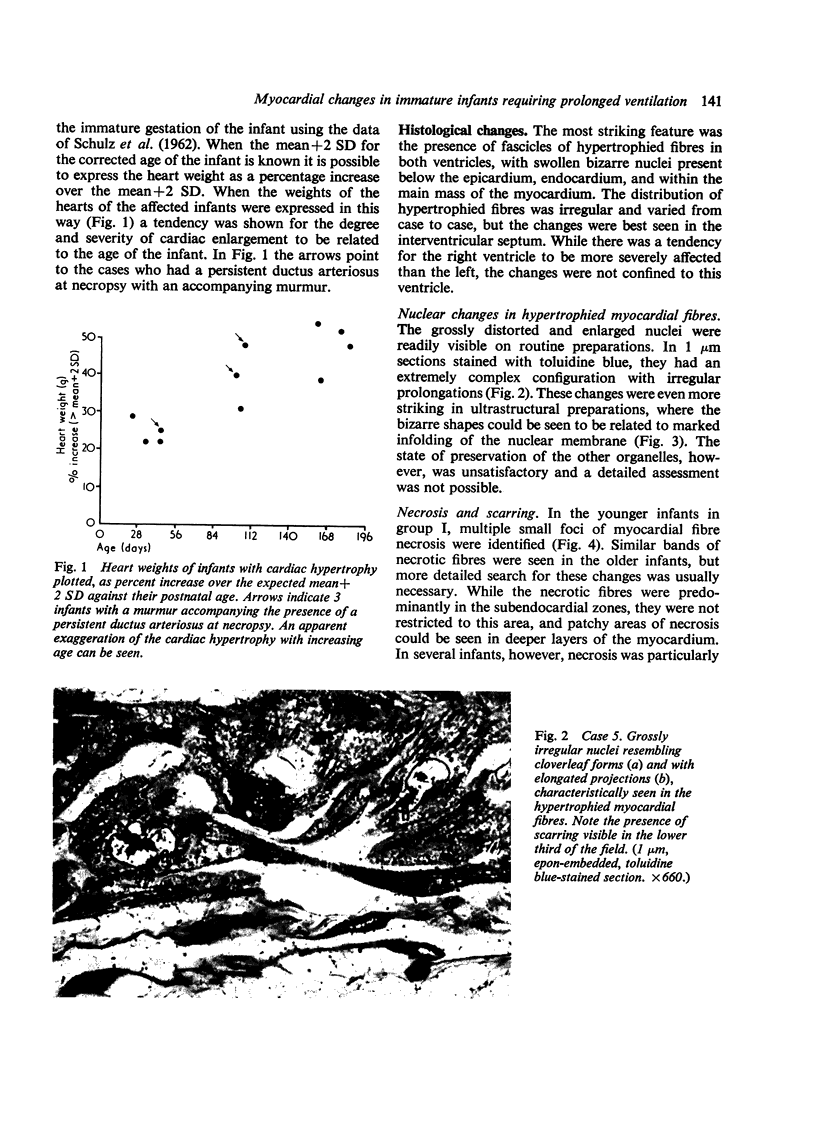
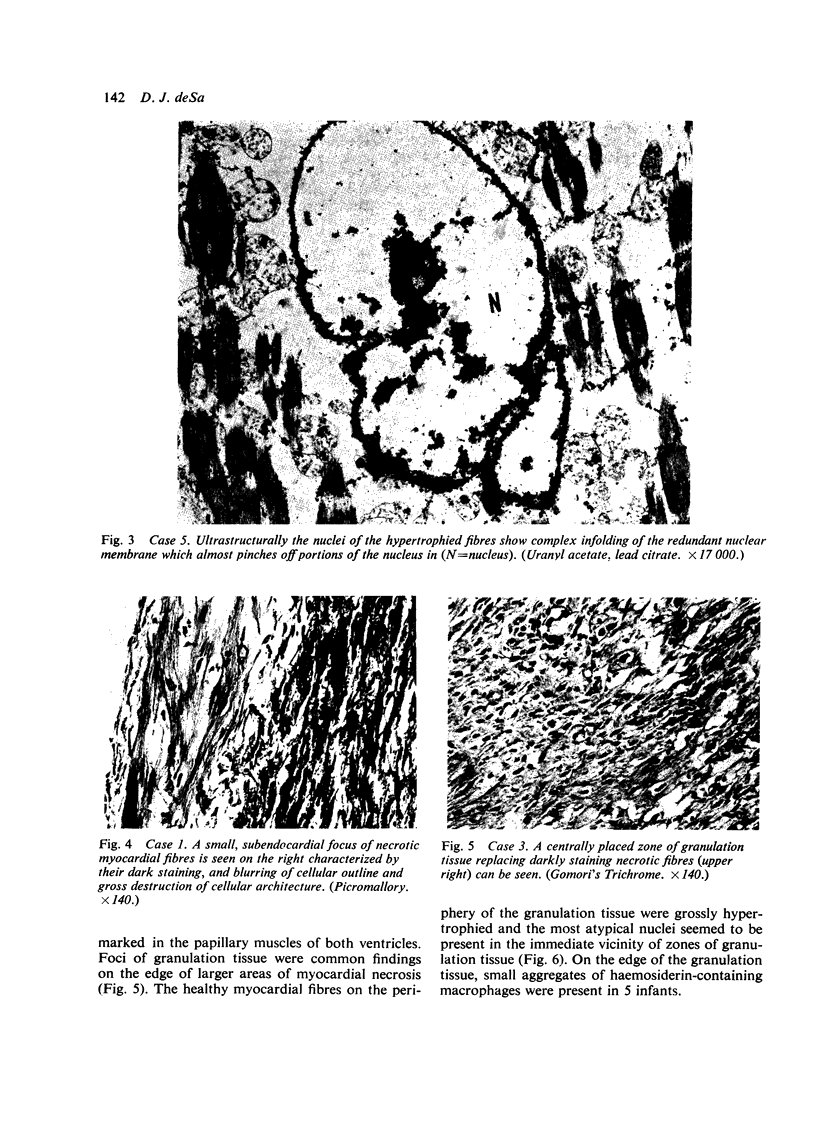
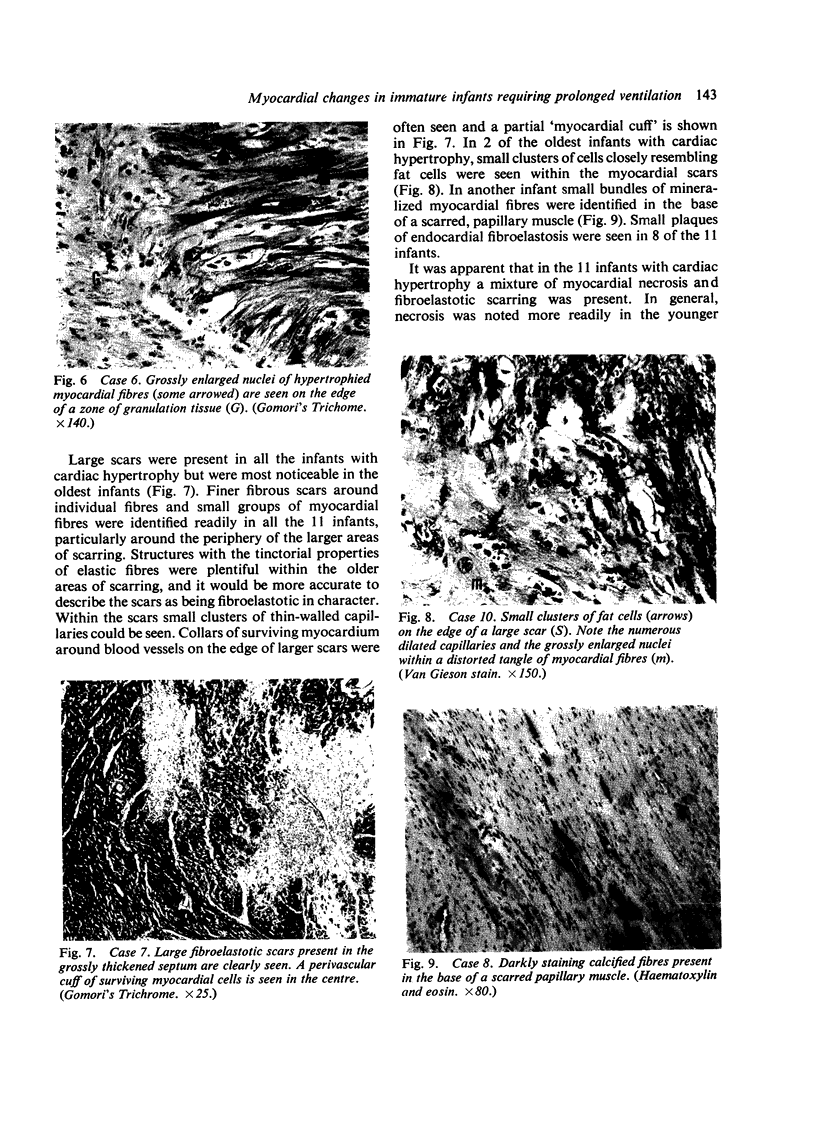
Fourteen infants who had required prolonged ventilation with high concentrations of oxygen for 14 days or more, were studied at necropsy. 11 infants of immature gestation at birth had gross cardiac hypertrophy, defined as a heart weight greater than 2 SD above the mean for their age. No congenital malformation of valves or septum was found, and in the 7 youngest infants with hypertrophy, the ductus arteriosus was anatomically patent. 3 of these 7 infants had an accompanying nurmur. All 11 infants had severe bronchopulmonary dysplasia. The cardiac hypertrophy affected both ventricles and septum in an irregular fashion and was associated with areas of necrosis, progressing to coarse fibroelastotic scars. The intramural vessels showed marked intimal thickening but the main coronary vessels were normal. The 2 youngest infants with cardiac hypertrophy showed the presence of intravascular and endocardial platelet thrombi. In the 3 infants without cardiac hypertrophy less severe zones of necrosis and scarring were present, and only occasional bundles of hypertrophied muscle fibres were seen. In an attempt to understand these hitherto undescribed lesions, a group of 50 fresh stillbirths and 50 first-week neonatal deaths of comparable gestational age were studied. In 19 of these infants foci of myocardial fibre necrosis were present. It is suggested that the lesions in the older infants represent a more advanced and continuing stage of that seen in the younger infants, and that the foci of necrosis are the result of continuing hypoxia and related problems to a failing coronary circulation. The possibility of myocardial damage represents a serious hazard to the successful therapy of the immature asphyxiated infants.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armiger L. C., Seelye R. N., Elswijk J. G., Carnell V. M., Benson D. C., Gavin J. B., Herdson P. B. Mitochondrial changes in dog myocardium induced by lactate in vivo. Lab Invest. 1975 Nov;33(5):502–508. [PubMed] [Google Scholar]
- Banerjee C. K., Girling D. J., Wigglesworth J. S. Pulmonary fibroplasia in newborn babies treated with oxygen and artificial ventilation. Arch Dis Child. 1972 Aug;47(254):509–518. doi: 10.1136/adc.47.254.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum G. L., Schwartz A., Llamas R., Castillo C. Left ventricular function in chronic obstructive lung disease. N Engl J Med. 1971 Aug 12;285(7):361–365. doi: 10.1056/NEJM197108122850701. [DOI] [PubMed] [Google Scholar]
- Cross K. W. Resuscitation of the asphyxiated infant. Br Med Bull. 1966 Jan;22(1):73–78. doi: 10.1093/oxfordjournals.bmb.a070442. [DOI] [PubMed] [Google Scholar]
- Ferrans V. J., Jones M., Maron B. J., Roberts W. C. The nuclear membranes in hypertrophied human cardiac muscle cells. Am J Pathol. 1975 Mar;78(3):427–460. [PMC free article] [PubMed] [Google Scholar]
- Fishman A. P. The left ventricle in "chronic bronchitis and emphysema". N Engl J Med. 1971 Aug 12;285(7):402–404. doi: 10.1056/NEJM197108122850710. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Seabra-Gomes R., Nayler W. G., Jennings R. B. Irreversible myocardial injury in anoxic perfused rat hearts. Am J Pathol. 1975 Sep;80(3):419–450. [PMC free article] [PubMed] [Google Scholar]
- Gelli M. G., Ericsson J. L., Enhörning G. ECG compared with myocardial ultrastructure in anoxic foetuses of normal and hyperglycaemic rabbits. Acta Paediatr Scand. 1968 Jul;57(4):330–338. doi: 10.1111/j.1651-2227.1968.tb07301.x. [DOI] [PubMed] [Google Scholar]
- Gennser G. Influence of hypoxia and glucose on contractility of papillary muscles from adult and neonatal rabbits. Biol Neonate. 1972;21(1):90–106. doi: 10.1159/000240499. [DOI] [PubMed] [Google Scholar]
- Guller B., Bozic C. Right-to-left shunting through a patent ductus arteriosus in a newborn with myocardial infarction. Cardiology. 1972;57(6):348–357. doi: 10.1159/000169533. [DOI] [PubMed] [Google Scholar]
- Haft J. I., Fani K. Intravascular platelet aggregation in the heart induced by stress. Circulation. 1973 Feb;47(2):353–358. doi: 10.1161/01.cir.47.2.353. [DOI] [PubMed] [Google Scholar]
- MICHELSON N. Bilateral ventricular hypertrophy due to chronic pulmonary disease. Dis Chest. 1960 Oct;38:435–446. doi: 10.1378/chest.38.4.435. [DOI] [PubMed] [Google Scholar]
- MacLeod D. P., Prasad K. Influence of glucose on the transmembrane action potential of papillary muscle. Effects of concentration, phlorizin and insulin, nonmetabolizable sugars, and stimulators of glycolysis. J Gen Physiol. 1969 Jun;53(6):792–815. doi: 10.1085/jgp.53.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron B. J., Ferrans V. J., Roberts W. C. Ultrastructural features of degenerated cardiac muscle cells in patients with cardiac hypertrophy. Am J Pathol. 1975 Jun;79(3):387–434. [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. R., Schwartz C. J. THE RELATION BETWEEN MYOCARDIAL LESIONS AND CORONARY ARTERY DISEASE II. A SELECTED GROUP OF PATIENTS WITH MASSIVE CARDIAC NECROSIS OR SCARRING. Br Heart J. 1963 Jan;25(1):1–24. doi: 10.1136/hrt.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northway W. H., Jr, Rosan R. C., Porter D. Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967 Feb 16;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- Rao B. S., Cohn K. E., Eldridge F. L., Hancock E. W. Left ventricular failure secondary to chronic pulmonary disease. Am J Med. 1968 Aug;45(2):229–241. doi: 10.1016/0002-9343(68)90041-7. [DOI] [PubMed] [Google Scholar]
- Ratliff N. B., Kopelman R. I., Goldner R. D., Cruz P. T., Hackel D. B. Formation of myocardial zonal lesions. Am J Pathol. 1975 May;79(2):321–334. [PMC free article] [PubMed] [Google Scholar]
- SCHULZ D. M., GIORDANO D. A., SCHULZ D. H. Weights of organs of fetuses and infants. Arch Pathol. 1962 Sep;74:244–250. [PubMed] [Google Scholar]
- Taghizadeh A., Reynolds E. O. Pathogenesis of bronchopulmonary dysplasia following hyaline membrane disease. Am J Pathol. 1976 Feb;82(2):241–264. [PMC free article] [PubMed] [Google Scholar]
- Tyson J. E., deSa D. J., Moore S. Thromboatheromatous complications of umbilical arterial catheterization in the newborn period. Clinicopathological study. Arch Dis Child. 1976 Oct;51(10):744–754. doi: 10.1136/adc.51.10.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenthal K. Studies of foetal mouse hearts in organ culture: metabolic requirements for prolonged function in vitro and the influence of cardiac maturation on substrate utilization. J Mol Cell Cardiol. 1973 Feb;5(1):87–99. doi: 10.1016/0022-2828(73)90038-2. [DOI] [PubMed] [Google Scholar]














