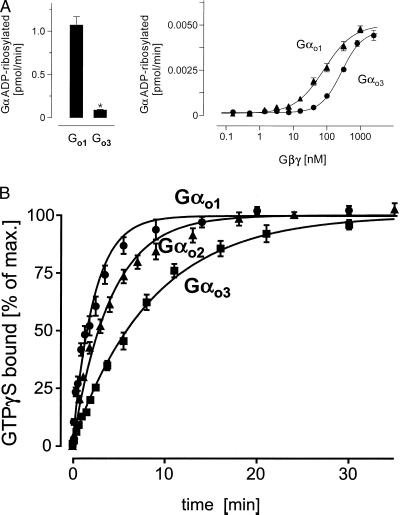
Figure 5.
(A) PT-mediated ADP ribosylation. Gαo isoforms (350 nM, Left; 4 nM, Right) were ADP-ribosylated in the presence of stoichiometric (Left) or increasing concentrations (Right) of Gβγ complexes using PT. The reactions were stopped by addition of an equal volume of 2× concentrated electrophoresis sample buffer and subjected to urea-SDS/PAGE. For quantification of 32P-incorporation dilutions of the reaction mixture were spotted on nitrocellulose membranes and dried. Gel slabs and membranes were autoradiographed and analyzed by using a PhosphorImager. Data shown are mean values ± SD (n = 3) from one typical experiment of three (∗, P < 0.01). (B) Time course of 35S-GTPγS binding to three purified Gαo isoforms. The appropriate Gα was added to the reaction mixture in a final concentration of 3–5 nM in the presence of 1 mM EDTA at 25°C. The binding reaction was carried out at 50 nM 35S-GTPγS, yielding a total binding of 110,000 to 180,000 cpm. The reaction was stopped at the indicated time points by diluting samples with ice-cold buffer followed by filtration through nitrocellulose. Filters were washed and counted in a liquid scintillator counter. Nonspecific binding was less than 5% of the total (3,600 to 7,800 cpm). Shown are mean values ± SD (n = 3) from one typical experiment of three.