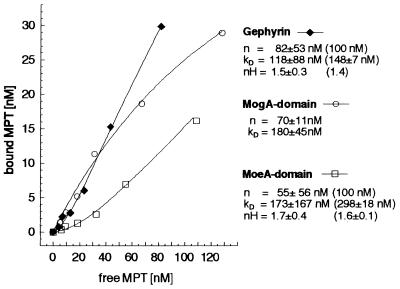
Figure 2.
Binding of MPT to gephyrin and to its two domains. The binding of 5–157 nM MPT, isolated from denatured xanthine oxidase, to 100 nM gephyrin (♦), MogA domain (○), and MoeA-domain (□) was analyzed by using gel filtration followed by HPLC analysis of Form A, the I2/KI-oxidation product of MPT, as described (13). Data were collected from binding experiments with six different MPT concentrations. Dissociation constants (Kd), maximal concentration of binding sites (n), and Hill coefficients (nH) are shown for each curve. In the case of cooperative types of binding (gephyrin, MoeA domain), no saturation with MPT was reached. Curve regressions with n = 100 nM (equimolar binding) gave Kd values and Hill coefficients that are shown in brackets.