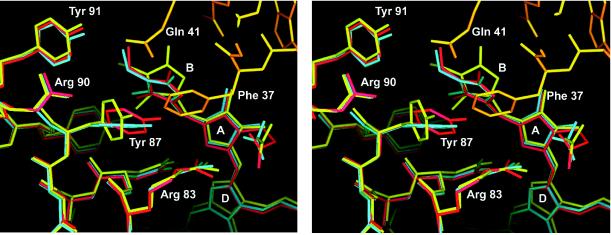
Figure 3.
Stereo representation of the interaction between the linker polypeptide and monomer 2. The Cα-aligned structures are shown for the symmetric, linker-free allophycocyanin (blue), monomer 3, which has no interaction with the linker polypeptide (red), and monomer 2 (yellow), which interacts with the N terminus of the long linker α-helix (orange). Water molecules are omitted for clarity. Phe-37 of the linker inserts between Tyr-87 and the pyrrole ring B of the corresponding β-subunit displacing both to opposite directions and disrupting the stacking interaction between the two aromatic ring systems, which is seen at the other two β-chromophores of the linker containing trimer. This figure was prepared in main (29).