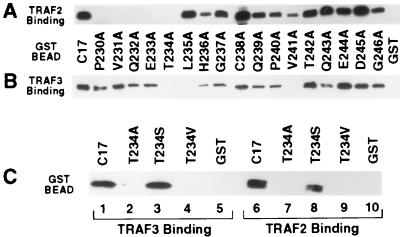
Figure 2.
Dissection of the CD40ct by alanine-scanning mutagenesis. (A and B) TRAF2 and TRAF3 contact overlapping but different sets of amino acid residues in the CD40ct. The same amount of GST protein and GST fusion proteins containing either wild-type C17 or one of 17 mutated forms of C17, with 1 aa residue replaced by alanine, was incubated with 293T cell extracts overexpressing Flu-tagged TRAF2 (A) or TRAF3 (B). The bound Flu-TRAF2 and Flu-TRAF3 were detected by Western blot analysis using anti-Flu antibody. (C) The hydroxyl group of the threonine at position 234 is essential for TRAF binding. The same amounts of GST protein and GST fusion proteins containing wild-type C17, T234A, T234S, and T234V were incubated with 293T cell extracts overexpressing Flu-tagged TRAF2 and TRAF3. The bound Flu-TRAF2 and Flu-TRAF3 were detected by Western blot analysis using anti-Flu antibody.