Abstract
Increasing environmental pollution in urban areas has been endangering the survival of carbonate stones in monuments and statuary for many decades. Numerous conservation treatments have been applied for the protection and consolidation of these works of art. Most of them, however, either release dangerous gases during curing or show very little efficacy. Bacterially induced carbonate mineralization has been proposed as a novel and environmentally friendly strategy for the conservation of deteriorated ornamental stone. However, the method appeared to display insufficient consolidation and plugging of pores. Here we report that Myxococcus xanthus-induced calcium carbonate precipitation efficiently protects and consolidates porous ornamental limestone. The newly formed carbonate cements calcite grains by depositing on the walls of the pores without plugging them. Sonication tests demonstrate that these new carbonate crystals are strongly attached to the substratum, mostly due to epitaxial growth on preexisting calcite grains. The new crystals are more stress resistant than the calcite grains of the original stone because they are organic-inorganic composites. Variations in the phosphate concentrations of the culture medium lead to changes in local pH and bacterial productivity. These affect the structure of the new cement and the type of precipitated CaCO3 polymorph (vaterite or calcite). The manipulation of culture medium composition creates new ways of controlling bacterial biomineralization that in the future could be applied to the conservation of ornamental stone.
The study of bacterially induced and mediated mineralization is an emerging interdisciplinary research area (see references 6, 28, and 32 for recent reviews on the topic). Bacterially induced precipitation of calcium carbonate, the so-called “carbonatogenesis” (21, 51), has drawn much attention in recent decades because of its numerous implications. These include (i) atmospheric CO2 fixation through carbonate sediment formation and lithification (22, 29, 36, 48, 49, 59, 65) and dolomite precipitation (76, 93), (ii) solid-phase capture of inorganic contaminants (95), (iii) the production of pathological concretions such as gallstones and kidney stones in humans (41, 44, 46), and (iv) understanding possible extraterrestrial biological processes like those of Martian carbonate-producing bacteria (58, 88). There is extensive literature on bacterial involvement in carbonate precipitation both in nature and in the laboratory (2, 14, 16, 19, 20, 21, 36, 40, 49, 57, 72). Recent research on bacterially induced carbonate precipitation has focused on a wide range of topics with important scientific and technological implications (7, 8, 38). One of them is the possible applications of this bacterial process to the conservation of stone artworks (63).
Architectural and sculptural stone undergoes deterioration due to physical, chemical, and/or biological weathering (69, 75, 77, 94, 97). The composition and textural characteristics of carbonate stones (limestones, dolostones, and marbles) make them particularly susceptible to weathering. Increasing atmospheric pollution has accelerated deterioration of carbonate stones in recent decades. Fossil fuel combustion resulting in acid rain-mediated mineral dissolution and sulfate crust development (69, 75, 97) is now jeopardizing the survival of monuments and statuary around the world (78). Many conservation treatments have been applied to the protection and consolidation of stone before extensive granular disintegration causes loss of surface material and therefore irreversible damage (4). Protection refers to treatments that waterproof and/or strengthen stone surfaces in order to keep water or other weathering agents from entering the core of the stone. Consolidation is the impregnation and thus strengthening of a friable decayed porous stone with a cementing and/or hardening product. Both treatments have been performed in the past using organic and inorganic materials (50), such as acrylic or epoxy resins (42) and Ba(OH)2 solutions (53). However, none of the treatments available to date have proven to be satisfactory. Organic treatments commonly result in the formation of incompatible, often harmful surface films. In addition, they generally release noxious solvents. Inorganic consolidation may be preferable since stone minerals and protective or consolidating materials share some physical-chemical affinity. For instance, the so-called limewater treatment (68) composed of Ca(OH)2 solutions, has been used to consolidate carbonate stones because calcium hydroxide easily carbonates in the presence of atmospheric CO2, resulting in calcite (CaCO3) formation. However, the limewater technique often leads to the formation of a superficial, micrometer-thick, friable aggregate of submicron-size calcite crystals and has an insufficient protection and/or consolidation effect (67).
Recently, bacterially induced carbonate mineralization has been proposed as an environmentally friendly method to protect decayed ornamental carbonate stone (3, 21, 63, 90). This new conservation method mimics what nature has been doing for eons, since many carbonate rocks have been cemented by microbe-induced calcium carbonate precipitation (16, 36). It has been known since Boquet et al. (14) that most heterotrophic soil bacteria induce calcium carbonate precipitation. A carbonate-producing soil bacterium (Bacillus cereus) was later applied as a conservation treatment for ornamental stone (21, 63). The method relies on the bacterially induced formation of a compatible carbonate precipitate on limestone, and unlike the limewater treatment, the carbonate cement is highly coherent (51). On the other hand, two important limitations of the initial, so-called CALCITE method (51) were its ineffectiveness for in-depth consolidation (apparently only a few microns) and the formation of a superficial film consisting of a mixture of biological remains. The latter plugged stone pores and provided no consolidation (90). It has been demonstrated that plugging accelerates decay (50). It is therefore recommended that stone treatments should leave the stone surface free to “breathe,” i.e., to allow vapor transfer. This has prevented the use of the bacteriogenic mineral plugging (using Bacillus pasteurii) technique proposed by Ferris and Stehmeier (35) for ornamental stone conservation, although variations of this technique have been used for sand bed consolidation (38, 85) and crack remediation in concrete (8, 70). Another potential drawback of the use of Bacillus in stone conservation is that these bacteria may form endospores which under appropriate conditions (i.e., temperature, humidity, and nutrient availability) may eventually lead to germination and uncontrolled bacteria growth and biofilm formation. The complexity and the large number of variables at work in bacterially induced carbonate precipitation (e.g., bacteria type, culture media, stone support, and application conditions) suggest that no general claim regarding the success or failure of the method can be made until further research has elucidated its potential and limits. It is therefore necessary to develop methods that will help to create a coherent carbonate cement in the porous system of the treated stone without at the same time blocking or plugging the pores. The method should also allow the biomineralization process to be stopped at will in order to avoid undesired side effects. This will lead to appropriate, controlled, and long-lasting protection and consolidation of decayed porous carbonate stones.
Here we report the development of a bacterially mediated carbonate precipitation method that can protect and consolidate porous carbonate stone. The selected microorganism, Myxococcus xanthus, is an abundant gram-negative, nonpathogenic aerobic soil bacterium. It belongs to a peculiar microbial group whose complex life cycle involves a remarkable process of morphogenesis and differentiation (30). Our research group demonstrated that M. xanthus induces the precipitation of carbonates, phosphates and sulfates (e.g., calcite, magnesium calcite, struvite, newberyite, schertelyte, and taylorite) in a wide range of solid and liquid media (9, 39, 40). It was however unknown whether the bacterium would be able to induce carbonate precipitation in a porous stone. Our aim in this paper is to determine the ability of M. xanthus to create a coherent protective and consolidating carbonate matrix in the porous system of limestone. Ultimately, we attempt to better understand bacterially induced carbonate precipitation on solid substrates in order to optimize and implement this biomineralization strategy as an effective conservation treatment.
MATERIALS AND METHODS
Carbonate stone.
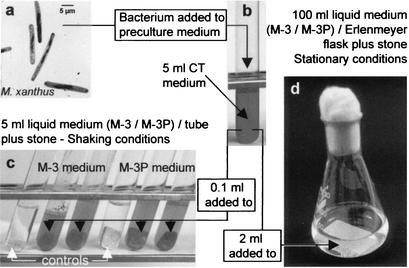
The support material was a porous limestone used extensively as a sculptural and architectural stone in Spanish historical buildings. A calcarenite composed of a mixture of benthic foraminifera, red algae, and fragments of bivalves and echinoderms (73), it is highly porous (24 up to 32%) and easily decays in urban environments. It develops gypsum crusts and granular disintegration due to the dissolution of sparry calcite cement and the crystallization of soluble salts (73, 75). Homogeneous stone blocks were selected and cut into two sizes: (i) 0.5 by 1 by 1 cm and (ii) 2.5 by 4.5 by 0.5 cm. The smallest samples were selected to represent optimal conditions (surface/volume ratio) for bacterially induced carbonate biomineralization. Furthermore, they were cultivated under shaking conditions to enhance bacterial growth (see below). Larger pieces were used to simulate a scenario closer to reality (in situ application), where a lower surface/volume ratio is expected (cultivated under stationary conditions; see below). Pieces were sterilized by flowing steam (tyndallization) for 1 h at 100°C. This process was performed four times in succession at 24-h intervals. Biomineralization tests were conducted in liquid media under constant shaking and stationary conditions. One small stone slab per test tube was used in experiments with shaking, whereas one large calcarenite slab per Erlenmeyer flask was used in stationary experiments (Fig. 1).
FIG. 1.
Schematic representation of the biomineralization experiments using M. xanthus: (a) Transmission electron photomicrographs of the bacterium. (b) Inoculum culture was incubated for 48 h at 28°C (shaking conditions). Incubation of the bacterial culture was carried out in test tubes (c) and Erlenmeyer flasks (d) containing calcarenite slabs immersed in M-3 or M-3P liquid media and incubated under shaking and stationary conditions, respectively.
Bacterial strain and culture media.
The microorganism used was M. xanthus (strain number 422 provided by the Spanish Type Culture Collection, Burjasot, Valencia, Spain). For inoculum preparation, M. xanthus was precultured in liquid medium CT (1% [wt/vol] Bacto Casitone and 0.1% [wt/vol] MgSO4 · 7H2O in a 10 mM phosphate buffer, pH 6.5). The culture was incubated on a shaker for 48 h at 28°C, which is the optimal duration for M. xanthus to reach a density of ∼2 × 109 cells/ml during the exponential growth (40). Tubes and Erlenmeyer flasks were sealed with Parafilm-covered cotton plugs after 1 week of incubation time in order to avoid excessive water evaporation during extended incubation periods. Oxygen availability was ensured by the large air reservoir in both the tubes and Erlenmeyer flasks. Liquid media M-3 [1% Bacto Casitone, 1% Ca(CH3COO)2 · 4H2O, 0.2% K2CO3 · 1/2H2O, in distilled water, pH 8] and M-3P [1% Bacto Casitone, 1% Ca(CH3COO)2 · 4H2O, 0.2% K2CO3 · 1/2H2O in a 10 mM phosphate buffer, pH 8] were used for biomineralization tests (Fig. 1). A pancreatic digest of casein (Bacto Casitone; Difco) was the nitrogen source in all media. Liquid media were sterilized by autoclaving for 20 min at 120°C.
Biomineralization experiments.
The set up for these experiments is shown in Fig. 1. Calcarenite slabs were placed in both M-3 and M-3P culture media (5 ml of culture medium in each test tube; 100 ml of culture medium in each Erlenmeyer flask) and inoculated with 0.1 ml (test tubes) and 2 ml (Erlenmeyer flasks) of M. xanthus inoculum culture. A minimum of three samples were run in each experiment. Test tubes were incubated at 28°C with constant shaking (160 rpm) using a rotary Certomat R shaker (Braun). Erlenmeyer flasks were incubated at 28°C without shaking. Control experiments identical to those indicated above were carried out without bacterial inoculation. Sterility tests of controls were performed by culturing 20-μl aliquots of culture media (collected following stone slab recovery) in CT solid medium (CT plus 1.8% purified agar-agar). The same procedure was used to check contamination of inoculated samples.
Weight increase through biomineralization.
Slabs subjected to bacterially induced mineralization were recovered at different times. pH was measured both at the start of the experiment and when recovering the slabs (with a Crison Micro pH 2001 device). Samples were rinsed three times with distilled water before drying at 37°C in a dark and dust-protected environment. Weight gain (average value of three samples) was calculated in terms of differences in weight between fresh and biomineralized calcarenite slabs at the end of incubation.
XRD analysis.
X-ray diffraction (XRD) was used to determine the mineralogy of the stone and newly formed carbonates. The diffractometer used was a Philips model PW-1710 with automatic slit, Cu Kα radiation (λ = 1.5405 Å), 3 to 60 °2θ explored area, and 0.05 °2θ s−1 goniometer speed. XRD goniometer calibration was performed using a silicon standard. Slab samples were placed in the XRD sample holder without any prior grinding or preparation. The limited solid residua which formed on the calcarenite blocks in the uninoculated media (control samples) were also analyzed.
SEM analysis.
Texture and penetration depth of the bacterially induced carbonates formed on the porous substratum were observed using scanning electron microscopy (SEM) (Zeiss DMS 950 electron microscope). Samples were gold coated prior to observation. Both stone surfaces in contact with the bacterial medium and sections perpendicular to the exposed surface were analyzed.
Porosimetry analysis.
Changes in stone porosity and pore size distribution following biomineralization were studied using mercury intrusion porosimetry (MIP) (with a Micromeritics Autopore 5510 device). Samples were dried overnight in a oven at 80°C prior to MIP analysis (for details, see reference 74).
Ultrasonic treatment.
Sonication is an effective means of cleaning or disrupting materials adhered to a surface (23, 56). Its effectiveness, measured as the amount of material removed from a surface, may give an indirect estimate of surface material-substratum adhesion force. In the case of carbonates precipitated on the calcarenite stones, sonication gives an estimate of both the newly formed carbonate adhesion force and the consolidation and/or protection efficacy of the newly formed carbonates. Large calcarenite slabs were collected following 30 days of incubation in the Erlenmeyer flasks. It was assumed that maximum carbonate precipitation was reached at this time. The samples were sonicated in deionized water for a duration of 5 min, five times in succession, using a 50-kHz ultrasonic bath (Ultrasons model, 200 W; J. P. Selecta). Samples were collected, dried for 24 h in an 80°C oven, and weighed after each 5-min sonication cycle. SEM was used to study the final appearance of the stone surfaces. Calcarenite samples of the same size that were not subjected to biomineralization were used as controls. One set of control samples was subjected to sonication, while another set was not sonicated. The latter samples were immersed in distilled water to estimate weight loss not due to sonication (i.e., calcite dissolution). A set of biomineralized samples was used to estimate weight loss due to dissolution (i.e., samples immersed in water but not sonicated). To minimize weight loss errors, only large samples were sonicated (i.e., because they have the smaller surface/volume ratio).
RESULTS
Weight changes.
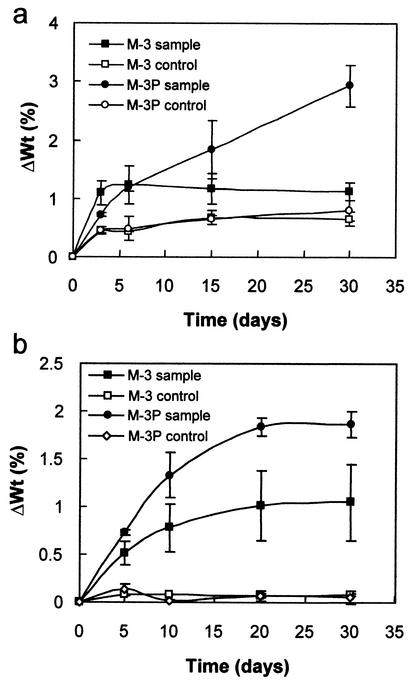
Increases of up to a few percent were observed in samples cultured under both shaking and stationary conditions (Fig. 2). Shaking enhanced weight gain due to favored bacterial growth and carbonate production (i.e., shaking ensured nutrient and metabolite exchange and facilitated aeration). The most significant increases were observed with samples cultured in M-3P medium, regardless of sample shape and experimental conditions. Maximum increases were found with the smaller samples (Fig. 2a), since their surface to volume ratio is greater. Larger samples displayed smaller weight increases, but weight gain trends in M-3 and M-3P media did not change significantly (Fig. 2b). These increases suggest that there is a significant amount of bacterially induced mineral precipitation. Figure 2 shows that this process was fastest over the first 5 to 10 days. Weight increases in controls were minimal and became insignificant with the smaller samples as of the third day.
FIG. 2.
Weight increase (ΔWt) versus time of small (a) and large (b) calcarenite slabs following bacterially induced carbonate mineralization. Error bars, standard deviations.
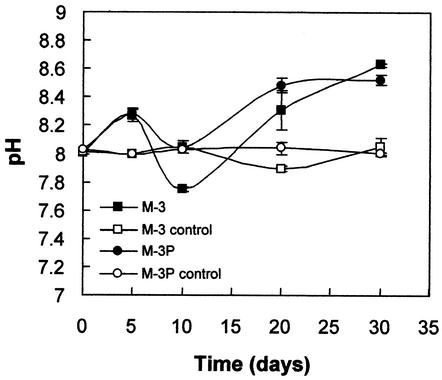
pH variations.
Fig. 3 shows pH values of culture media following collection of the large calcarenite slabs at different time intervals. Both inoculated M-3 and M-3P media underwent an increase in pH from 8 up to 8.5 to 8.7 over the 30-day span of the experiment. pH rapidly increased in the first 5 days, undergoing a significant reduction at the 10th day (when weight gain rates start to decrease; see Fig. 2). The pH drop is associated with the maximum rate of carbonate precipitation (see Discussion). pH fluctuations were more significant in M-3 than in M-3P media. This is consistent with the presence of a phosphate buffer in the latter. Controls underwent no significant pH variations.
FIG. 3.
pH variations versus time for M-3 and M-3P liquid culture media (controls refer to uninoculated media with calcarenite slabs). Error bars, standard deviations.
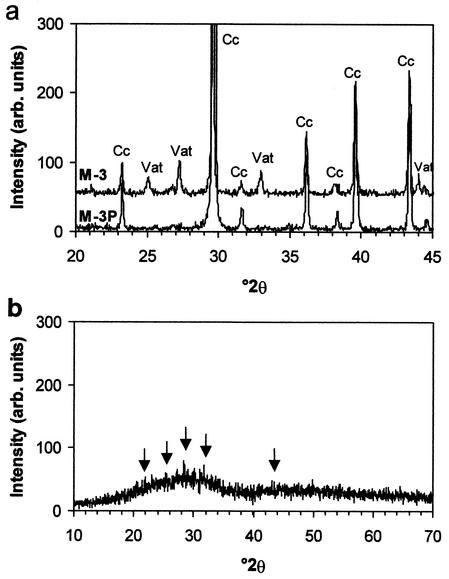
XRD analysis.
Small quantities of vaterite were detected. Vaterite formed in M-3-cultured samples and, in smaller quantities, in the large samples cultured in M-3P medium under stationary conditions (Fig. 4a). Vaterite is a rare metastable calcium carbonate polymorph (54) and in our experiments was clearly a bacterially induced newly formed phase since no vaterite was detected in either the raw calcarenite or controls. Calcite was the main phase in both controls and samples cultured in M-3 and M-3P media (Fig. 4a). This is consistent with the composition of the stone, which is a pure limestone. Any calcite produced by bacterial activity was masked by the calcite that already existed in the calcarenite. Precipitates on the walls of the vessels that contained uninoculated control samples displayed minimal quantities of poorly crystalline hydroxylapatite [Ca5(PO4)3(OH)] as well as an undetermined amorphous phase (Fig. 4b). The latter phase is evidenced by a broad XRD reflection with high background noise.
FIG. 4.
XRD patterns of calcarenite slabs subjected to bacterially induced carbonate mineralization tests (abbreviations: Cc, calcite; Vat, vaterite) (a) and solid residua formed in uninoculated culture media (both M-3 and M-3P) (b). Arrows indicate hydroxylapatite diffraction peaks. Goniometer calibration was performed using a silicon standard. Bragg peak identification was performed using JPDF files. Cu Kα radiation, λ = 1.5406 Å.
SEM analysis. (i) Small samples cultured under conditions of constant shaking.
In small samples cultured under conditions of constant shaking, the control calcarenite showed sparitic calcite crystals which constitute the carbonate cement in this limestone (Fig. 5a). Bacterial cells fully enclosed by calcium carbonate can be observed in samples treated with inoculated M-3 medium (Fig. 5b). Underlying these bodies is a porous network of acicular vaterite crystals that cements calcarenite grains without blocking or filling the pores. These crystals are observed down to nearly 1 mm in the porous system of the stone. Samples in inoculated M-3P medium developed a coarse sparitic cement composed of calcite rombohedra. This cement fully covered the pore walls down to a depth of 0.5 mm (Fig. 5c). When grown on large preexisting calcite crystals, the newly formed calcite rombohedra show a preferred crystallographic orientation (Fig. 5d). Holes (diameter, circa 2 μm) can be observed in the newly formed calcitic cement blanket. These holes appear to be the casts of former bacteria. Control samples displayed no newly formed carbonates because of the absence of M. xanthus inoculation.
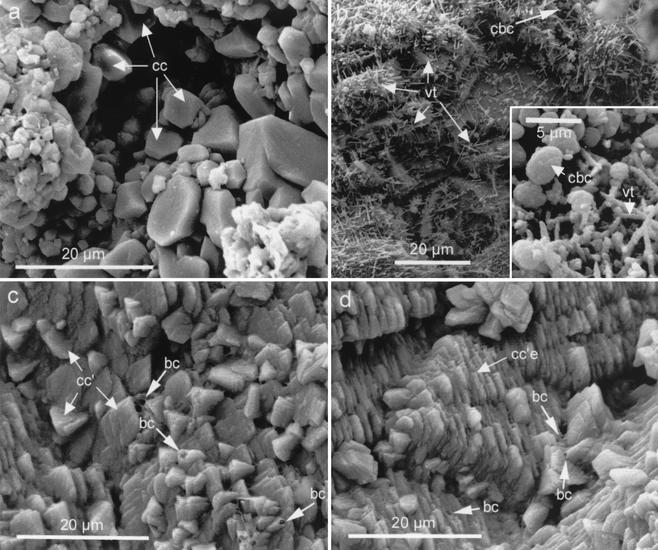
FIG. 5.
SEM photomicrographs of small samples cultured under shaking conditions. (a) Representative image of control calcarenite. Sparitic calcite (cc) crystals are indicated by arrows (note that microtextural features of sterilized calcarenite controls and of controls immersed in uninoculated M-3 or M-3P media are identical). (b) Stone sample subjected to biomineralization in the M-3 medium, showing calcified bacterial cells (cbc) and needle-like vaterite (vt) crystals (inset shows a magnified view of the upper-right corner). (c) Newly formed calcite rhombohedra (cc′) on calcarenite cultured in the M-3P medium (bacterial casts [bc] are also indicated). (d) Newly formed calcite crystals developing epitaxially (cc′e) on preexisting calcite crystals and showing preferred crystallographic orientation (bacterial casts are also indicated).
(ii) Large samples cultured under stationary conditions.
As in the previous case, no carbonate precipitation took place in the controls when large samples were cultured under stationary conditions. After 5 days of immersion in the inoculated M-3 medium, abundant calcified bacteria cells developed in association with a network of newly formed fibrous carbonate aggregates (i.e., vaterite crystals). The latter linked the calcarenite grains without blocking the pores in the stone. This is similar to what was observed in Fig. 5b. Calcified bacterial cells fully covered the walls of the pores after 30 days of culturing in the M-3 medium (Fig. 6a). The formation of calcite casts of bacterial cells linked by needle-shaped vaterite crystals was observed after 5 days of incubation time in the M-3P medium (Fig. 6b). These crystals cemented the calcarenite as in Fig. 5b. However, a film with numerous drying cracks, which confirm its organic nature, deposited on the walls of the pores (Fig. 6c). Nonetheless, no pore blocking occurred, even after an incubation time of 30 days. At this time, SEM observations showed significant amounts of calcified bacterial cells and newly formed vaterite fibers which cemented the calcarenite carbonate grains (Fig. 6d).
FIG. 6.
SEM photomicrographs of large samples cultured under nonshaking conditions. (a) Calcified bacterial cells (cbc) covering the pore walls of samples cultivated in the M-3 medium (after 30 days). (b) Detail of calcified bacteria linked by needle-like vaterite (vt) crystals in samples cultured in the M-3P medium (after 10 days). (c) Sample cultured in the M-3P medium (after 30 days) showing incipient development of an EPS film (the arrow indicates cracks in the film; note that no pore plugging occurred). (d) Massive needle-like vaterite crystals blanketing stone pores following 30 days of culture in the M-3P medium. Calcified bacterial cells are also observed covering the pore walls.
No contamination was observed in the controls regardless of the stone slab size. Viability tests demonstrated the growth of M. xanthus following inoculation onto CT solid medium at the end of the experiments. M. xanthus growth occurred regardless of the medium type and the size of the cultured stone samples. The development of either myxospores or fruiting bodies was not observed either in the inoculated cultured media or on the cultured stone slabs.
(iii) Large samples submitted to sonication.
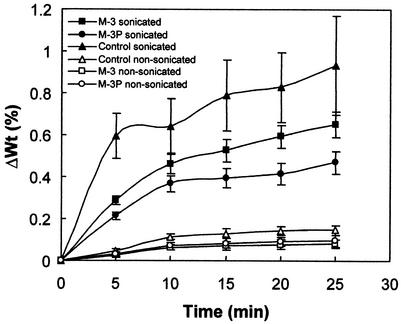
Sonication induced significant damage in the controls. Fissuring and spalling of calcite grains along the rombohedral {104} cleavage planes was systematic (Fig. 7a) and evidenced the dramatic effect of the test. However, samples subjected to bacterially induced mineralization showed much less damage, and the newly formed carbonate grains were not removed in detectable amounts. The organic film in samples cultivated in the M-3P medium was almost entirely removed. However, the calcified bacterial cells and vaterite needles remained intact (Fig. 7b). Sonication uncovered sparitic calcite cement (Fig. 7c) as a result of film removal from samples cultivated in M-3P medium. The film appeared to have covered the newly formed rombohedral calcite crystals, thus preventing their observation. The sparitic calcite rombohedra were identical to those formed on small samples subjected to biomineralization in test tubes under shaking conditions (Fig. 5c and d). Figure 7d shows an SEM photomicrograph in which the partially removed film still covers some of the newly formed sparitic cement. Carbonated bacterial cells in samples cultivated in the M-3 medium and subjected to sonication were also preserved. As in the former case, almost no film survived the sonication process. Selective removal of the organic film, which developed primarily after the 10th day of a biomineralization experiment, demonstrates its poor adhesion to the substrate. SEM observations of samples submitted to sonication are consistent with the smaller weight losses found in the samples subjected to bacterially induced mineralization compared with the sonicated controls (Fig. 8). Samples subjected to biomineralization in the M-3P medium showed minimum weight losses, values very close to those of controls immersed in deionized water but not sonicated. Samples cultured in the M-3 medium showed weight loss values between those of sonicated controls and those incubated in M-3P medium. SEM observations and weight loss measurements of sonicated samples demonstrate the strong adhesion between the stone and the newly formed carbonates, as well as the positive consolidating and/or protecting effect of the biomineralization (maximum in the case of M-3P medium). They also demonstrate that the newly formed carbonates are more resistant to physical-mechanical stresses than the calcite in the stone. In the newly formed calcite crystals, sonication produced no cracking along the {104} rombohedral cleavage planes, while in the crystals of the calcarenite it did. Figure 8 also shows evidence of a weight loss reduction (if compared with controls) in biomineralized samples immersed in water but not submitted to sonication.
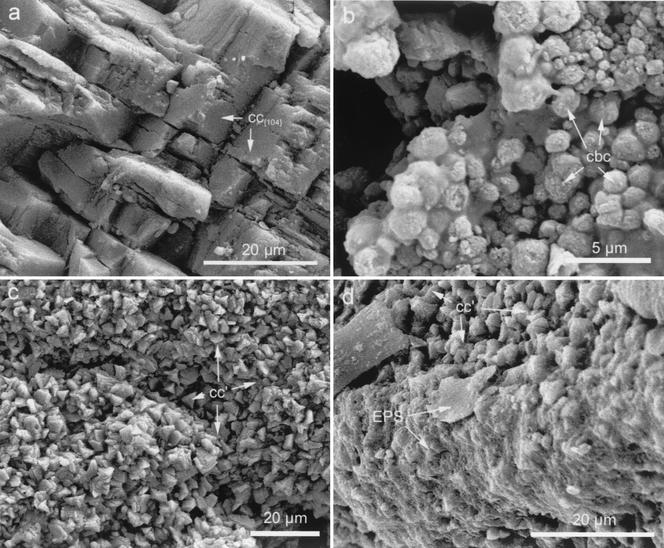
FIG. 7.
SEM photomicrographs of samples subjected to sonication. (a) Control (i.e., calcarenite slab not submitted to biomineralization but sonicated) showing spalling and fissuring of calcite crystals along rhombohedral {104} cleavage planes (cc{104}). (b) Calcified bacteria (cbc) blanketing stone samples cultured in the M-3P medium. (c) Newly formed calcite (cc′) rombohedra blanketing the calcarenite. (d) Detail of a calcarenite showing a partially removed EPS film (EPS) which covered the newly formed calcite cement (cc′).
FIG. 8.
Calcarenite slab weight loss (ΔWt) versus sonication time. One set of biomineralized samples (cultured in inoculated M-3 or M-3P media for 30 days) was sonicated, and the other was not (i.e., was only immersed in deionized water). Two control sets were tested (i.e., calcarenite slabs not subjected to biomineralization): calcarenite slabs (i) not sonicated (control nonsonicated) and (ii) sonicated (control sonicated). Error bars, standard deviations.
Porosimetry analysis.
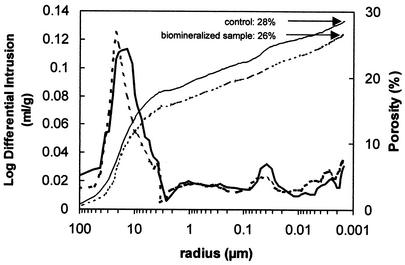
MIP results showed no significant changes in porosity or pore size distribution when comparing treated and untreated (control) samples (Fig. 9). Apparently this technique is not accurate enough to detect the slight porosity reductions induced by vaterite and/or calcite (and the eventual film) growth on the pore walls of treated samples. On the other hand, it is difficult to determine whether the detected porosity variations were due to newly formed carbonates or to intrinsic changes in porosity. In fact, open porosity (accessible to Hg) in the raw material ranged from 24 to 32%, while treated stones had porosity values between 24% and 26%.
FIG. 9.
Representative MIP plots showing both the cumulative intrusion curves (i.e., porosity) and pore size distribution curves [i.e., log of differential intrusion, or log(dv/dr), versus r, where v is the intruded volume and r is the pore radius] for fresh (solid line) and biomineralized (dotted line) stone samples.
DISCUSSION
Calcium carbonate precipitation.
Calcite (and vaterite) precipitation in solution occurs via the following overall equilibrium reaction (87):
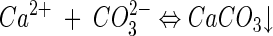 |
(1) |
The thermodynamic solubility product of calcite is 3.31 × 10−9 M2 while that of vaterite is 1.22 × 10−8 M2 (45). The higher solubility of vaterite (1 order of magnitude higher than that of calcite) accounts for its lower stability (54). Carbonate ions are produced in water by the following equilibrium reactions, which are strongly pH dependent (87):
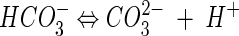 |
(2) |
 |
(3) |
It follows from these reactions that an increase in CO32− concentration occurs under alkaline conditions, thereby favoring calcite precipitation. This is particularly relevant when studying bacterially induced carbonate precipitation, as will be discussed below.
In our study abiogenic precipitation of calcium carbonate (i.e., with no direct or indirect involvement of bacteria) has been ruled out, since no carbonate crystallized in the controls. All of our observations suggest that carbonate precipitation occurred in association with bacterial activity. Microbially induced CaCO3 precipitation is a far more complex process than abiogenic precipitation (85). In fact, different mechanisms accounting for carbonate formation induced by bacteria have been reported (31). Aerobic heterotrophic bacteria like M. xanthus may play both a direct and an indirect role in carbonate precipitation. Because of their high surface area-to-volume ratio, production of chemically active polymers, surface properties, and reliance on diffusion, bacteria acting as a highly reactive geochemical interface (52, 81) play a direct role in carbonate precipitation. Biofilms formed by bacteria and their extracellular polymeric substances (EPS) (25), are particularly effective at binding ions from solution and act as heterogeneous nucleation surfaces for mineral deposition (11, 12, 15, 17, 22, 34, 47, 60, 81). It has been demonstrated that calcium carbonate precipitates on M. xanthus cellular membranes (39). Défarge et al. (26) also demonstrated that microscopic three-dimensional organic networks inherited from sheaths of dead cyanobacteria and from other EPS of microbes acted as a matrix for calcification. They reported that crystal nucleation began at acidic sites which are capable of binding a wide range of cations. Both gram-positive and gram-negative cell walls contain a number of surface functional groups such as carboxyl, hydroxyl, and phosphate sites. These groups deprotonate and become increasingly negative at alkaline pH, thereby displaying a strong electrostatic affinity for aqueous metal cations (10, 33). Ca2+ is not likely to be used in large quantities by intracellular microbial metabolic processes, and therefore, it accumulates outside the cell where it is preferentially adsorbed (82). Plette et al. (66) demonstrated substantive Ca2+ binding to carboxylic and phosphatic sites of isolated Rhodococcus erythropolis cell walls. Once attached to cell walls, Ca2+ can bond to carbonate ions, thus resulting in the formation of CaCO3. On the other hand, various bacterial metabolic activities, such as carbon dioxide fixation, nitrate reduction or oxidative deamination of amino acids, may indirectly induce calcium carbonate precipitation. This is brought about by locally changing the physical-chemical parameters (e.g., increases in pH and ionic strength) in the environment in which bacteria live (5, 18, 20, 40, 83, 85). In this manner precipitation of carbonates occurs even if the overall system is undersaturated with respect to these phases (64), since supersaturation is reached locally in the surrounding of the bacterial cells. Simple solution chemistry cannot apply in this microenvironment because there is a fluid-solid interface in which thermodynamic activation energies are drastically reduced and chemical reactions resulting in carbonate precipitation become possible (89).
M. xanthus metabolic activity involves production of NH3 by means of oxidative deamination of amino acids. NH3 creates an alkaline microenvironment around the cell according to the following reaction (43):
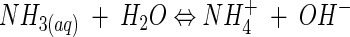 |
(4) |
Reaction 4 is consistent with the pH rise we observed from an initial value of 8 to final values of 8.5 to 8.7 (Fig. 3).
Metabolic activity and the resulting local pH rise may lead to a high local supersaturation with respect to either vaterite or calcite (see reactions 1 to 3). CO2 is also produced by the bacteria. CO2 dissolves and transforms into either HCO3− or CO32− at higher pH. In our experiments additional carbonate ions were supplied by K2CO3 present in the culture media. Once sufficient supersaturation is achieved, calcium carbonate formation by heterogeneous nucleation occurs on the bacterial cell walls, as can be observed in the SEM photomicrographs showing bacterial casts within rombohedral calcite crystals. Carbonate precipitation leads to a reduction in pH, a fact consistent with the data shown in Fig. 2 and 3. In fact, the pH drop takes place when the rate of calcium carbonate precipitation (i.e., weight gain) is close to its maximum. The incorporation of Ca2+ and CO32− in the newly formed carbonates brings about the pH reduction (87). Since the bacteria are attached to the carbonate grains of the stone, the growth of the newly formed carbonate creates a cement that adheres strongly to the substratum, as demonstrated by the sonication tests. This new cement commonly grows epitaxially on previous calcite crystals, thus matching their crystallographic orientation (13). The latter is consistent with our observations of preferred crystallographic orientation of newly formed calcite rombohedra which develop on large sparitic calcite crystals (Fig. 5d). On the other hand, heterogeneous nucleation also took place on the stone surface, not in contact with, but in the proximity of bacterial cells. The needle-like vaterite crystals which appear in Fig. 5b are evidence of this.
Vaterite is a metastable polymorph of CaCO3 with a hexagonal structure (P63/mmc space group) and is rare in natural environments (54). It is unstable and rapidly transforms into calcite (or aragonite) at room temperature in an aqueous solution (84). However, it commonly forms in synthetic processes and has often been reported to develop in the presence of microorganisms in nature (37). The presence of microbial films and the apparent affinity of the bacterial cell walls for vaterite appear to cause vaterite precipitation in a manner similar to that described by Mann et al. (55). These authors induced vaterite formation on a stearic acid monolayer, which appeared to mimic a cell membrane. In this case the vaterite was formed through electrostatic and stereochemical interactions at the inorganic-organic interface. Dalas et al. (24) also induced vaterite precipitation on polymeric structures that have a large amount of carboxylate groups. In our study a similar mechanism seems to be at work, since vaterite was detected surrounding calcified bacterial cells and also in areas in which EPS films were found to cover the carbonate grains. One complementary explanation for vaterite development in the presence of M. xanthus is suggested by Ostwald's step rule (43). According to this rule, stable phase formation is sometimes preceded by the formation of metastable phases which are normally favored under nonequilibrium conditions (i.e., high supersaturation). Vaterite could therefore be a precursor of calcite. It forms in both M-3P and M-3 culture media in localized areas in which there is a relatively high supersaturation due to a rapid rise in pH. The latter increase could be a consequence of local intense metabolic bacterial activity. Local increases in metabolic activity are consistent with the localized vaterite formation at high supersaturation in the M-3P medium and also with the idiomorphic rombohedral calcite crystals formed nearby at a lower supersaturation. A high supersaturation seems to be a prerequisite for vaterite formation in the laboratory (84). This is consistent with our observation of vaterite habit, size, and crystal density. The large number of tiny acicular vaterite crystals is in agreement with their formation under highly supersaturated conditions, while large, well-formed rombohedral calcite crystals are formed at relatively low supersaturation. Mullin (62) previously described the details of supersaturation influence on nucleation density, crystal size, and crystal morphology.
As already indicated, differences existed in the type of calcium carbonate polymorph formed in the two tested culture media and in the crystal yield. The only compositional difference between M-3 and M-3P media is the phosphate buffer present in the latter. The phosphate buffer seems to have a profound effect both on the bacterial cell yield and on the carbonate productivity as well as on the supersaturation preceding the nucleation of carbonate crystals. Phosphorous is essential for bacterial development. Phosphate was added to the medium for growing the inoculum and could be present in small but sufficient quantities to ensure bacterial growth in M-3 medium over the first 5 to 10 days of the tests. Little bacterial activity occurred in M-3 medium after this initial period, and the carbonate precipitation resulted in limited weight increases (Fig. 2). This was most likely due to phosphorous depletion. Conversely, phosphorous was more readily available in the M-3P medium, thus ensuring greater and longer-lasting bacterial activity. This is consistent with the greater and continuous weight increase over the 30-day duration of the test. A greater bacterial production also led to a higher yield in carbonate crystals. On the other hand, the buffering effect of the phosphate prevented rapid local pH variations and departures from equilibrium, at least during the initial days of the test (Fig. 3). Low supersaturation values therefore led to a low nucleation density (62) and the formation of calcite crystals that display equilibrium morphologies, like euhedral rombohedra. The unconstrained pH variations in the M-3 medium could, however, cause rapid increases in CO32− concentration, thus resulting in high supersaturation and massive vaterite formation, followed by a significant reduction in pH (Fig. 3). It has been reported that ammonia released by bacteria causes continuous increases in pH. This in turn increases the rate of carbonate precipitation. There is, however, a pH threshold above which a reduction in the microbial count can be observed (38; A. Kantzas, G. F. Ferris, K. N. Jha, and F. M. Mourits, unpublished data presented at the CIM Annual Technical Conference, 1992). Thus, an optimal pH (around 8.5) exists which leads to maximum bacterially induced carbonatogenesis. The phosphate buffer in the M-3P medium seems to have prevented any substantial departure from the optimal pH during a longer period of time.
Consolidating and/or protecting role of bacterially induced carbonate crystals.
The deposition of calcite and vaterite crystals strongly adhered to pore surfaces ensures a protective and/or consolidating effect following M. xanthus induced carbonate precipitation. The newly formed carbonates were more resistant to mechanical stress (i.e., sonication) than the calcite crystals in the stone. Newly formed carbonates may incorporate a certain amount of organic molecules produced by bacteria. Organic molecules may harden calcite, as occurs in echinoderms or bivalves (1). Calcite crystals in echinoderms are tougher, do not crack along rombohedral {104} cleavage planes, and show a conchoidal fracture which is typical of hard glasses or metals. Strengthening is due to the incorporation of organics which form a composite material (1, 96). This type of hardening is consistent with observations of bacterially produced calcite rombohedra which show no homogeneous extinction (90). Apparently, the presence of organic molecules causes a misalignment of different domains within a single crystal. Further research should be undertaken to directly observe these crystallographic features in calcite formed by bacterial processes (i.e., by using transmission electron microscopy). In addition, bacterially induced calcite crystals are assumed to be more resistant to dissolution (i.e., acid attack), since it has been experimentally demonstrated that biomineralized calcite is less soluble than inorganically precipitated calcite (61). The results shown in Fig. 8 also indicate slight weight loss reduction in biomineralized samples immersed in water but not sonicated when compared to the controls. This is crucial if stones are to be protected in polluted environments. On the other hand, the newly formed carbonates strongly adhere to the substrate, leading to significant reduction in stone weight loss following sonication. Samples cultured in the M-3P medium display the lowest weight loss upon sonication, a result which can be accounted for by the epitaxial nature of most of the newly formed calcite cement. The above-mentioned consolidating and/or protecting effects are indeed significant, because bacteria induce carbonate cementation to a depth of several hundred micrometers (≥500 μm). Most importantly, no plugging or blocking of pores takes place during this cementation. No accurate data currently exist regarding the depth of penetration of bacterially induced carbonatogenesis. Castanier et al. (21) reported that the bioconsolidating cement was a few micrometers thick but did not specify the depth of the effect.
Plugging of stone pores has been one of the main drawbacks to using bacterially induced carbonatogenesis as a new conservation alternative (90). Plugging is mainly a consequence of EPS film formation (79, 90). This film has sometimes been misinterpreted as a newly formed carbonate deposit (51). We found that limited EPS film formation occurred on stones in M-3P medium and that only small quantities occurred in M-3 medium in stationary cultures. It should be noted that organic films are unable to attach to the stones under shaking conditions. At any rate, this limited film development causes no pore plugging.
Damage to stone has been related to bacterial biofilms (91). It has been suggested that biofilms are optimal media for further bacteria colonization because they retain humidity and provide a number of nutrients (94). Most of the bacteria commonly associated with biofilms that develop on stone surfaces are heterotrophic (80), although chemolithotrophic bacteria have been described in a number of cases (86). The role of heterotrophic bacteria in stone decay is, however, considered limited by some authors (27), particularly when compared with chemolithotrophic bacteria (86). Biofilms have also been suggested to play a role in carbonate precipitation because they adsorb ions and provide nucleation sites (17, 25, 52). Reitner et al. (71) even concluded that carbonates were associated not with bacterial calcification in microbialites but rather with EPS calcification. However, in our experiments we observed carbonate precipitation in the absence of organic films under shaking conditions. Yet, sonication tests did result in the selective removal of EPS films, which points to their poor adhesion to the substrate and to the absence of any calcification in them.
Concluding remarks on bacterially induced consolidation and/or protection of stone.
We have demonstrated that M. xanthus is able to produce a coherent carbonate cement. An important advantage to the use of this bacterium is that it does not complete its life cycle in the tested culture media. No dormant stage, i.e., myxospore formation, was observed in the tested culture media. Although at the end of the experiment viability tests demonstrated that M. xanthus was still alive in the liquid culture media, the drying of the stone sample led to bacterial death (i.e., no fruiting bodies were observed). Vegetative cell death can prevent eventual uncontrolled bacterial growth. By contrast, Bacillus subtilis and B. pasteurii caused biofilm formation and plugging of pores in biomineralization tests (90). The fact that M. xanthus dies can be used to the conservator's advantage in the protection and consolidation of stone artworks. Weight gain results suggest that most carbonate precipitation occurs in the first 5 to 10 days, which will be particularly relevant for real-case conservation interventions (e.g., logistic and economic implications). There was no significant weight gain in M-3 medium after 5 to 10 days, which suggests that in situ bacterial mineralization treatments of architectural or sculptural stone need not last longer than 5 to 10 days. Although in M-3P medium there was some weight gain as late as 20 to 30 days, the most significant carbonate production occurred over the first 5 to 10 days. In order to stop the biomineralization process, the supply of culture media should be discontinued, thus constraining the negative effects of organic film growth.
It has thus been demonstrated that culture medium composition, culture conditions, and type of bacteria are key factors for controlling EPS film formation and for the enhancement of the consolidation and protection effects of the newly formed carbonate cement.
Concerning the possible interaction between autochthonous microbiota and a bacterium used for biomineralization, Le Métayer-Levrel et al. (51) found that, after bacterially induced carbonatogenesis in ornamental stone, no increases in the bacterial activity or changes in the autochthonous microbiota were observed, nor was an increase in autochthonous bacterial activity observed immediately after treatment or 4 years later. Furthermore, Urzi et al. (92) demonstrated that the most common bacteria isolated in building stones (e.g., Geodermatophilus sp. and Microccocus sp.) induce carbonate precipitation under laboratory conditions. They could, therefore, act synergistically in the biomineralization process when nutrients are added upon a bacterial treatment. Future research should explore whether addition of the inoculated culture media tested actually contributes synergistically to the protection and consolidation of ornamental stone.
Our study demonstrates that biomineralization induced by bacterial activity results in significant protection and consolidation of porous carbonate stones used in sculptural and architectural heritage. However, much research has yet to be conducted in order to properly exploit this new conservation methodology. Future work should be done using other culture media and M. xanthus mutants that could improve consolidation. Other stone supports, including noncarbonate stones like granites or sandstones, should also be studied. We have performed preliminary tests demonstrating the ability of M. xanthus to develop on silicate supports, such as silica glass or ceramics, where it also induces carbonate precipitation—results which further the possibilities of using this biomineralization treatment on silicate rocks. Finally, in situ small-scale testing of bacterial biomineralization in ornamental calcareous stones should be performed before larger-scale applications are undertaken in buildings and sculptures.
Acknowledgments
This work was supported by the Spanish Dirección General de Investigación (DGI contracts MAT2001-3074 and BOS2001-3285) and by Research Groups NMR 0179 and FQM 195 of the Junta de Andalucía.
We thank Belén Fernández Luque and Concepción Jiménez López for their help with the biomineralization experiments and the weight gain measurements. The CIC personnel of the University of Granada also assisted in the SEM analyses. Editing of the English version of the manuscript was done by Marco Bettini. The thorough revision and comments by two anonymous referees are greatly appreciated.
REFERENCES
- 1.Addadi, B. L., and S. Weiner. 1992. Control and design principles in biological mineralization. Angew. Chem. Int. Ed. Engl. 31:153-169. [Google Scholar]
- 2.Adolphe, J. M., and C. Billy. 1974. Biosynthèse de calcite par une association bactérienne. C. R. Acad. Sci. Paris 278:2873-2874. [Google Scholar]
- 3.Adolphe, J. M., J. F. Loubière, J. Paradas, and F. Soleilhavoup. September 1990. Procédé de traitement biologique d'une surface artificielle. European patent 90400G97.0 (after French patent 8903517, 1989).
- 4.Ashurst, J., and F. G. Dimes. 1990. Conservation of building and decorative stone. Butterworth-Heinemann, London, United Kingdom.
- 5.Bachmeier, K. L., A. E. Williams, J. R. Warmington, and S. S. Bang. 2002. Urease activity in microbiologically-induced calcite precipitation. J. Biotechnol. 93:171-181. [DOI] [PubMed] [Google Scholar]
- 6.Banfield, J. F., and K. H. Nealson (ed.). 1997. Geomicrobiology: interactions between microbes and minerals. Reviews in mineralogy, vol. 35. Mineralogical Society of America, Washington, D.C.
- 7.Banfield, J. F., S. A. Welch, and K. J. Edwards. 1998. Microbes as geochemical agents. Newsl. Geochem. Soc. 96:11-17. [Google Scholar]
- 8.Bang, S. S., J. K. Galinat, and V. Ramakrishnan. 2001. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzyme Microb. Technol. 28:404-409. [DOI] [PubMed] [Google Scholar]
- 9.Ben Omar, N., M. T. González-Muñoz, and J. M. Arias. 1998. Struvite crystallization on Myxococcus cells. Chemosphere 36:475-481. [Google Scholar]
- 10.Beveridge, T. J., and R. G. E. Murray. 1980. Sites of metal deposition in the cell walls of Bacillus subtilis. J. Bacteriol. 141:876-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beveridge, T. J., J. D. Meloche, W. S. Fyfe, and R. G. E. Murray. 1983. Diagenesis of metals chemically complexed to bacteria: laboratory formation of metal phosphates, sulfides and organic condensates in artificial sediments. Appl. Environ. Microbiol. 45:1094-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beveridge, T. J., S. A. Makin, J. L. Kadurugamuwa, and Z. Li. 1997. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 20:291-303. [DOI] [PubMed] [Google Scholar]
- 13.Bloss, F. D. 1971. Crystallography and crystal chemistry: an introduction. Holt, Rinehart and Winston, New York, N.Y.
- 14.Boquet, E., A. Boronat, and A. Ramos-Cormenzana. 1973. Production of calcite (calcium carbonate) crystals by soil bacteria is a common phenomenon. Nature 246:527-529. [Google Scholar]
- 15.Brown, D. A., D. C. Kamineni, J. A. Sawicki, and T. J. Beveridge. 1994. Minerals associated with biofilms occurring on exposed rock in a granitic underground research laboratory. Appl. Environ. Microbiol. 60:3182-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buczynski, C., and H. S. Chafetz. 1991. Habit of bacterially induced precipitates of calcium carbonate and the influence of medium viscosity on mineralogy. J. Sediment. Petrol. 61:226-233. [Google Scholar]
- 17.Camoin, G. F., P. Gautret, L. F. Montaggioni, and G. Cabioch. 1999. Nature and environmental significance of microbialites in Quaternary reefs: the Tahiti paradox. Sediment. Geol. 126:271-304. [Google Scholar]
- 18.Cañaveras, J. C., M. Hoyos, S. Sanchez-Moral, E. Sanz-Rubio, J. Bedoya, V. Soler, I. Groth, P. Schumann, L. Laiz, I. Gonzalez, and C. Saiz-Jimenez. 1999. Microbial communities associated with hydromagnesite and needle-fiber aragonite deposits in a karstic cave (Altamira, Northern Spain). Geomicrobiol. J. 16:9-25. [Google Scholar]
- 19.Castanier, S., A. Maurin, and A. Bianchi. 1984. Participation bactérienne à la précipitation du carbonate. C. R. Acad. Sci. Paris 299:1333-1336. [Google Scholar]
- 20.Castanier, S., G. Le Métayer-Levrel, and J. P. Perthuisot. 1999. Ca-carbonates precipitation and limestone genesis—the microbiologist point of view. Sediment. Geol. 126:9-23. [Google Scholar]
- 21.Castanier, S., G. Le Métayer-Levrel, G. Orial, J. F. Loubière, and J. P. Perthuisot. 2000. Bacterial carbonatogenesis and applications to preservation and restoration of historic property, p. 201-216. In O. Ciferri, P. Tiano, and G. Mastromei (ed.), Of microbes and art: the role of microbial communities in the degradation and protection of cultural heritage. Plenum, New York, N.Y.
- 22.Chafetz, H. S., and C. Buczynski. 1992. Bacterially induced lithification of microbial mats. Palaios 7:277-293. [Google Scholar]
- 23.Crawford, A. E. 1963. A practical introduction to ultrasonic cleaning. Ultrasonics 1:65-69. [Google Scholar]
- 24.Dalas, E., P. Klepetsanis, and P. G. Koutsoukos. 1999. The overgrowth of calcium carbonate on poly(vinylchloride-covinyl acetate-co-maleic acid). Langmuir 15:8322-8327. [Google Scholar]
- 25.Decho, A. W. 2000. Microbial biofilms in intertidal systems: and overview. Continent. Shelf Res. 20:1257-1273. [Google Scholar]
- 26.Défarge, C., J. Trichet, A. M. Jaunet, M. Robert, J. Tribble, and F. J. Sansone. 1996. Texture of microbial sediments revealed by cryo-scanning electron microcopy. J. Sediment. Res. 66:935-947. [Google Scholar]
- 27.Descheemaeker, P., and J. Swings. 1995. The application of fatty acid methyl ester analysis (FAME) for the identification of heterotrophic bacteria present in decaying Lede Stone of the St. Bavo Cathedral in Ghent. Sci. Total Environ. 167:241-247. [Google Scholar]
- 28.Douglas, S., and T. J. Beveridge. 1998. Mineral formation by bacteria in natural microbial communities. FEMS Microbiol. Ecol. 26:79-88. [Google Scholar]
- 29.Drew, G. H. 1914. On the precipitation of calcium carbonate in the sea by marine denitrifying bacteria. Carnegie Inst. Wash. Publ. 1825:9-45. [Google Scholar]
- 30.Dworkin, M., and D. Kaiser. 1993. Myxobacteria II. American Society for Microbiology, Washington D.C.
- 31.Ehrlich, H. L. 1999. Microbes as geologic agents: their role in mineral formation. Geomicrobiol. J. 16:135-153. [Google Scholar]
- 32.Ehrlich, H. L. 2002. Geomicrobiology, 4th ed. Marcel Dekker, New York, N.Y.
- 33.Fein, J. B. 2000. Quantifying the effects of bacteria on adsorption reactions in water-rock systems. Chem. Geol. 169:265-280. [Google Scholar]
- 34.Ferris, F. G., W. S. Fyfe, and T. J. Beveridge. 1988. Metallic ion binding by Bacillus subtilis: implications for the fossilization of microorganisms. Geology 16:149-152. [Google Scholar]
- 35.Ferris, F. G., and L. G. Stehmeier. September 1992. Bacteriogenic mineral plugging. U.S. patent 5,143,155.
- 36.Folk, R. 1993. SEM imaging of bacteria and nannobacteria in carbonate sediments and rocks. J. Sediment. Petrol. 63:990-999. [Google Scholar]
- 37.Giralt, S., R. Julià, and J. Klerkx. 2001. Microbial biscuits of vaterite in lake Issyk-Kul (Republic of Kyrgyzstan). J. Sediment. Petrol. 71:430-435. [Google Scholar]
- 38.Gollapudi, U. K., C. L. Knutson, S. S. Bang, and M. R. Islam. 1985. A new method for controlling leaching through permeable channels. Chemosphere 30:695-705. [Google Scholar]
- 39.Gonzalez-Muñoz, M. T., N. Ben Omar, M. Martínez-Cañamero, M. Rodriguez-Gallego, A. Lopez Galindo, and J. M. Arias. 1996. Struvite and calcite crystallization induced by cellular membranes of Myxococcus xanthus. J. Crystal Growth 163:434-439. [Google Scholar]
- 40.Gonzalez-Muñoz, M. T., K. B. Chekroun, A. B. Aboud, J. M. Arias, and M. Rodriguez-Gallego. 2000. Bacterially induced Mg-calcite formation: Role of Mg2+ in development of crystal morphology. J. Sediment. Petrol. 70:559-564. [Google Scholar]
- 41.Hall, A., and J. D. Taylor. 1971. The occurrence of vaterite in gastropod egg-shells. Min. Mag. 38:521-525. [Google Scholar]
- 42.Horie, C. V. 1987. Materials for conservation: organic consolidants, adhesives and coatings. Butterworths, London, United Kingdom.
- 43.Jiménez-López, C., E. Caballero, F. J. Huertas, and C. S. Romanek. 2001. Chemical, mineralogical and isotope behavior, and phase transformation during the precipitation of calcium carbonate minerals from intermediate ionic solutions at 25°C. Geochim. Cosmochim. Acta 65:3219-3231. [Google Scholar]
- 44.Kajander, E. O., and N. Çiftçioglu. 1998. Nanobacteria: an alternative mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc. Natl. Acad. Sci. USA 95:8274-8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanakis, J., and E. Dalas. 2000. The crystallization of vaterite on fibrin. J. Crystal Growth 219:277-282. [Google Scholar]
- 46.Keefe, W. E. 1976. Formation of crystalline deposits by several genera of the family Enterobacteriaceae. Infect. Immun. 14:590-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konhauser, K. O., S. Schultze-Lam, F. G. Ferris, W. S. Fyfe, F. J. Longstaffe, and T. J. Beveridge. 1994. Mineral precipitation by epilithic biofilms in the Speed River, Ontario, Canada. Appl. Environ. Microbiol. 60:549-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krumbein, W. E. 1974. On the precipitation of aragonite on the surface of marine bacteria. Naturwissenchaften 61:167.. [DOI] [PubMed] [Google Scholar]
- 49.Krumbein, W. E. 1979. Photolithotrophic and chemoorganotrophic activity of bacteria and algae as related to beachrock formation and degradation (Gulf of Aqaba, Sinai). Geomicrobiol. J. 1:139-203. [Google Scholar]
- 50.Lazzarini, L., and M. Tabasso. 1986. Il restauro della pietra. Cedam, Padua, Italy.
- 51.Le Métayer-Levrel, G., S. Castanier, G. Orial, J. F. Loubière, and J. P. Perthuisot. 1999. Applications of bacterial carbonatogenesis to the protection and regeneration of limestones in buildings and historic patrimony. Sediment. Geol. 126:25-34. [Google Scholar]
- 52.Léviellé, R. J., W. S. Fyfe, and F. J. Longstaffe. 2000. Geomicrobiology of carbonate-silicate microbialites from Hawaiian basaltic sea caves. Chem. Geol. 169:339-355. [Google Scholar]
- 53.Lewin, S. Z., and N. S. Baer. 1974. Rationale of the barium hydroxide-urea treatment of decayed stone. Studies Conserv. 19:24-35. [Google Scholar]
- 54.Lippmann, F. 1973. Sedimentary carbonate minerals. Springer-Verlag, Berlin, Germany.
- 55.Mann, S., B. R. Heywood, S. Rajam, and J. D. Birchall. 1988. Controlled crystallization of CaCO3 under steric acid monolayers. Nature 334:692-695. [Google Scholar]
- 56.Masselin, I., X. Chesseray, L. Durand-Bourlier, J. M. Lainé, P. Y. Syzaret, and D. Lemondant. 2001. Effects of sonication on polymeric membranes. J. Membr. Sci. 181:213-220. [Google Scholar]
- 57.McCallum, M. F., and K. Guhathakurta. 1970. The precipitation of calcium carbonate from seawater by bacteria isolated from Bahama bank sediments. J. Appl. Bacteriol. 33:649-655. [DOI] [PubMed] [Google Scholar]
- 58.McKay, D. S., E. K. Gibson, K. L. Thomas-Keprta, H. Vali, C. S. Romanek, S. Clement, X. D. F. Chillier, C. R. Maechling, and R. N. Zare. 1996. Search for past life on Mars: Possible relic biogenic activity in Martian meteorite ALH84001. Science 273:924-930. [DOI] [PubMed] [Google Scholar]
- 59.Monger, H. C., L. A. Daugherty, W. C. Lindemann, and C. M. Liddell. 1991. Microbial precipitation of pedogenic calcite. Geology 19:997-1000. [Google Scholar]
- 60.Morita, R. Y. 1980. Calcium carbonate precipitation by marine bacteria. Geomicrobiol. J. 2:63-82. [Google Scholar]
- 61.Morse, J. W. 1983. The kinetics of calcium carbonate dissolution and precipitation, p. 227-264. In R. J. Reeder (ed.), Carbonates: mineralogy and chemistry. Reviews in mineralogy, vol. 11. Mineralogic Society of America, Washington, D.C.
- 62.Mullin, J. W. 1992. Crystallization, 3rd ed. Butterworth, London, United Kingdom.
- 63.Orial, G., S. Castanier, G. Le Métayer-Levrel, and J. F. Loubiere. 1993. The biomineralization: a new process to protect calcareous stone applied to historic monuments, p. 98-116. In H. Ktoishi, T. Arai, and K. Yamano (ed.), Proceedings of the 2nd International Conference on Biodeterioration of Cultural Property. International Communications Specialists, Tokyo, Japan.
- 64.Párraga, J., M. A. Rivadeneyra, R. Delgado, J. Iñiguez, M. Soriano, and G. Delgado. 1998. Study of biomineral formation by bacteria from soil solution equilibria. React. Function. Polymers 36:265-271. [Google Scholar]
- 65.Pedley, M. 1992. Freshwater (phytoherm) reefs: the role of biofilms and their bearing on marine reef cementation. Sediment. Geol. 79:255-274. [Google Scholar]
- 66.Plette, A. C. C., M. F. Benedetti, and W. H. Riemsdijk. 1996. Competitive binding of protons, calcium, cadmium, and zinc to isolated cell walls of Gram-positive bacterium. Environ. Sci. Technol. 30:1902-1910. [Google Scholar]
- 67.Price, C., K. Ross, and G. White. 1988. A further appraisal of the ′lime technique' for limestone consolidation, using a radioactive tracer. Studies Conserv. 33:178-186. [Google Scholar]
- 68.Price, C. A. 1985. The consolidation of limestone using a lime poultice and limewater. Bayerisches Denkmalpflege Arbeitsh. 31:148-151. [Google Scholar]
- 69.Price, C. A. 1996. Stone conservation. An overview of current research. Getty Conservation Institute, Los Angeles, Calif.
- 70.Ramachandran, S. K., V. Ramakrishnan, and S. S. Bang. 2001. Remediation of concrete using microorganisms. ACI Mater. J. 98:3-9. [Google Scholar]
- 71.Reitner, J., P. Gautret, F. Marin, and F. Neuweiler. 1995. Automicrites in a modern microbialite. Formation model via organic matrices (Lizard Island, Great Barrier Reef, Australia). Bull. Inst. Océanogr. Monaco 14:237-263. [Google Scholar]
- 72.Rivadeneyra, M. A., G. Delgado, A. Ramos-Cormenzana, and R. Delgado. 1998. Biomineralization of carbonates by Halomonas eurihalina in solid and liquid media with different salinities: crystal formation sequence. Res. Microbiol. 149:277-287. [DOI] [PubMed] [Google Scholar]
- 73.Rodriguez-Navarro, C. 1998. Causas y mecanisnos de alteración de los materiales calcáreos de las Catedrales de Granada y Jaén. Ph.D. thesis. Universidad de Granada, Granada, Spain.
- 74.Rodriguez-Navarro, C., and E. Sebastian. 1995. Técnicas de análisis del sistema poroso de materiales pétreos ornamentales: usos y limitaciones. Ing. Civil 96:130-142. [Google Scholar]
- 75.Rodriguez-Navarro, C., and E. Sebastian. 1996. Role of particulate matter from vehicle exhaust on porous building stones (limestone) sulfation. Sci. Total Environ. 187:79-91. [Google Scholar]
- 76.Rodriguez-Navarro, C., E. Sebastian, and M. Rodriguez-Gallego. 1997. An urban model for dolomite precipitation: authigenic dolomite on weathered building stones. Sediment. Geol. 109:1-11. [Google Scholar]
- 77.Rodriguez-Navarro, C., and E. Doehne. 1999. Salt weathering: influence of evaporation rate, supersaturation and crystallization pattern. Earth Surface Processes Landforms 24:191-209. [Google Scholar]
- 78.Rodriguez-Navarro, C., and E. Doehne. 1999. Time-lapse video and ESEM microscopy: integrated tools for understanding processes in-situ. Am. Lab. 31:28-35. [Google Scholar]
- 79.Ross, N., R. Villemur, L. Deschênes, and R. Samson. 2001. Clogging of limestone fracture by stimulating groundwater microbes. Water Res. 35:2029-2037. [DOI] [PubMed] [Google Scholar]
- 80.Saiz-Jimenez, C. 1994. Biodeterioration of stone in historic buildings and monuments, p. 587-604. In G. C. Llewellyn (ed.), Biodeterioration research 4. Plenum Press, New York, N.Y.
- 81.Schultze-Lam, S., D. Fortin, B. S. Davis, and T. J. Beveridge. 1996. Mineralization of bacterial surfaces. Chem. Geol. 132:171-181. [Google Scholar]
- 82.Silver, S., K. Toth, and H. Scribner. 1975. Facilitated transport of calcium by cells and subcellular membranes of Bacillus subtilis and Escherichia coli. J. Bacteriol. 12:880-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simkiss, K., and K. M. Wilbur. 1991. Biomineralization. Cell biology and mineral deposition. Academic Press, London, United Kingdom.
- 84.Spanos, N., and P. G. Koutsoukos. 1998. The transformation of vaterite to calcite: effect of the conditions of the solutions in contact with the mineral phase. J. Crystal Growth 191:783-790. [Google Scholar]
- 85.Stocks-Fischer, S., J. K. Galinat, and S. S. Bang. 1999. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 31:1563-1571. [Google Scholar]
- 86.Strzelczyk, A. B. 1981. Stone, p. 61-80. In A. H. Rose (ed.), Microbial biodeterioration. Economic microbiology, vol. 6. Academic Press, London, United Kingdom.
- 87.Stumm, W., and J. J. Morgan. 1981. Aquatic chemistry. Wiley-Interscience, New York, N.Y.
- 88.Thomas-Keprta, K. L., D. S. McKay, S. J. Wentworth, T. O. Stevens, A. E. Taunton, C. C. Allen, A. Coleman, E. K. Gibson, and C. S. Romanek. 1998. Bacterial mineralization patterns in basaltic aquifers: implications for possible life in Martian meteorite ALH84001. Geology 26:1031-1034. [DOI] [PubMed] [Google Scholar]
- 89.Thompson, J. B., and F. G. Ferris. 1990. Cyanobacterial precipitation of gypsum, calcite, and magnesite from natural alkaline lake water. Geology 18:995-998. [Google Scholar]
- 90.Tiano, P., L. Biagiotti, and G. Mastromei. 1999. Bacterially bio-mediated calcite precipitation for monumental stones conservation: methods of evaluation. J. Microbiol. Methods 36:139-145. [DOI] [PubMed] [Google Scholar]
- 91.Urzi, C., S. Lisi, G. Criseo, and A. Pernice. 1991. Adhesion and degradation of marble by a Microccocus strain isolated from it. Geomicrobiol. J. 9:81-90. [Google Scholar]
- 92.Urzi, C., M. Garcia-Valles, M. Vendrell, and A. Pernice. 1999. Biomineralization processes on rock and monument surfaces observed in field and laboratory conditions. Geomicrobiol. J. 16:39-54. [Google Scholar]
- 93.Vasconcelos, C., J. A. McKenzie, S. Bernasconi, D. Grujic, and A. J. Tien. 1995. Microbial mediation as a possible mechanism for natural dolomite formation at low temperatures. Nature 377:220-222. [Google Scholar]
- 94.Wakefield, R. D., and M. S. Jones. 1998. An introduction to stone colonizing micro-organisms and biodeterioration of building stone. Q. J. Eng. Geol. 31:301-313. [Google Scholar]
- 95.Warren, L. A., P. A. Maurice, N. Parmar, and F. G. Ferris. 2001. Microbially mediated calcium carbonate precipitation: implications for interpreting calcite precipitation and for solid-phase capture of inorganic contaminants. Geomicrobiol. J. 18:93-115. [Google Scholar]
- 96.Weiner, S., L. Addali, and H. D. Wagner. 2000. Materials design in biology. Mater. Sci. Eng. C 11:1-8. [Google Scholar]
- 97.Winkler, E. M. 1994. Stone in architecture. Springer-Verlag, Berlin, Germany.